Changes in thermodynamic functions during adsorption. Thermodynamics of adsorption
Interaction of polymers with liquids and gases
The processes of interaction of polymers with low molecular weight liquids play an important role in the processes of formation of finished products (for example, fibers from a solution), modification of the properties (plasticization) of the material, as well as in the operating conditions of these products in various liquid environments. The interaction is expressed in the absorption of liquid by the polymer and is called sorption. If sorption occurs in the volume of a polymer material, it is called absorption. If absorption occurs in surface layers, then the process is called adsorption.
Sorption
The adsorption mechanism is due to the presence of surface tension forces at the interfaces between media (Fig. 5.1) due to the difference in the forces of intermolecular interaction between them. This leads to the accumulation of excess energy on the surface of the substance, which tends to draw in its surface molecules (molecules adsorbent) and weaker interacting molecules (molecules adsorptive) inside the volume. The amount of adsorption largely depends on the specific surface area of the adsorbent. Numerically, adsorption is expressed by the number of moles of adsorbed substance per unit mass of adsorbent - x/m.
The study of sorption allows one to obtain valuable information about the structure of the polymer and the degree of packing of its molecules.
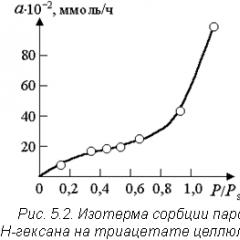
Typically, sorption processes are described using curves of the dependence of the amount of adsorbed substance on its concentration (or pressure) in the gas phase at a constant temperature (sorption isotherms, Fig. 5.2.). Here the value R/R s is the ratio of the vapor pressure of the adsorbent to the pressure of its saturated vapor at a given temperature.

In the region of low vapor pressures, Henry's linear law is satisfied:
Where A- amount of adsorbed substance; a m- limiting adsorption, proportional to the active surface of the adsorbent; p- sorbate pressure; k- adsorption constant. In Fig. 5.2, the completion of monomolecular adsorption is determined by the exit of the sorption isotherm to a shelf in the relative pressure range of 0.4 ÷ 0.5.
In the presence of polymolecular adsorption and condensation on the surface of a porous adsorbent ( R/R s > 0.6 in Fig. 5.2) use the universal equation
 | (5.3) |
Thermodynamics of the adsorption process
Since, as a rule, the intermolecular interaction of adsorbent molecules is less intense than that of the adsorbent, adsorption occurs with a decrease in the surface free energy (Δ F < 0) и выделением тепла (уменьшением энтальпии ΔN < 0). При равновесии процессов адсорбции и десорбции ΔF= 0. The value calculated during the adsorption process characterizes the number and activity of groups on the surface of the adsorbent that are capable of reacting with the absorbent. During adsorption, the entropy of the system also decreases (Δ S < 0), поскольку молекулы абсорбтива ограничивают подвижность молекул полимера, уменьшая возможное число конформаций: ΔS = k ln( W 2 / W 1), where is Boltzmann’s constant, W 2 and W 1 - thermodynamic probability of the final and initial state of the system.
Current page: 6 (book has 19 pages total) [available reading passage: 13 pages]
Font:
100% +
34. Nature of adsorption forces
The interaction between adsorbent molecules with the surface of the adsorbent during the so-called. physical adsorption can be due to various reasons. Then the potential that determines the interaction of one adsorbent molecule with one atom of a nonpolar adsorbent can be expressed as follows:
θ = −Cr 6 +Br 12 ,
where r is the distance between the centers of particles; C – dispersion attraction constant; B is a constant that characterizes the energy of repulsive forces.
It is quite obvious that at relatively distant distances the forces of attraction should prevail, and at close distances the forces of repulsion should prevail. Also, at certain distances these forces must be equal, which will correspond to a minimum of free energy. But it is important to note that during adsorption, dispersion forces act simultaneously between each non-polar particle.
Since the interaction energy of particles can quickly decrease with distance, to determine the potential of adsorption forces it is sufficient to carry out the summation on the nearest atoms of the adsorbent. It is important that during the adsorption of complex nonpolar molecules, the potential energy can be approximately calculated as the sum of all potential adsorption energies of the molecule units.
If the adsorbent consists of ions, then the action of the already known dispersion forces can be supplemented by the action of the inductive forces of attraction of dipoles that are induced in the molecules of the adsorbent by the electric field, which, in turn, is created by the ions of the adsorbent lattice.
With such an interaction, the share of inductive forces in the adsorption interaction can be proportional to the polarizability of the adsorbent molecule and the square of the field strength on this adsorbent surface.
If the adsorption of polar molecules of the adsorbent occurs on a polar adsorbent, then the dipoles in this case polarize the atoms of the adsorbent, i.e., they seem to induce electrical moments in them. Due to this influence, the inductive interaction is added to the dispersion interaction.
The inductive interaction itself is usually small and, depending on the dipole of the adsorbent molecule and the polarizability of the adsorbent, can reach large values. If molecules are adsorbed on an adsorbent that has ions or dipoles on the surface, the so-called interaction of ions or dipoles of the adsorbent with the electrostatic field of the adsorbent itself.
In this case, the molecules of the adsorbent can even be oriented in the field of the adsorbent, and orientational Coulomb interaction occurs. It usually happens that the energies of inductive and orientational interactions are less than the energy of dispersive interactions, and therefore it is accepted that the energy of intermolecular attraction is determined by the energy of dispersive attraction.
Adsorption can also be caused by the formation of a hydrogen bond. A bond of this type can occur during adsorption on adsorbents that contain hydroxyl groups of molecules such as water, alcohols, ammonia and amines on the surface. When a hydrogen bond is formed, the energy of interaction between the adsorbent and the adsorbent can be quite large, and the heat that is released during such adsorption is significantly greater than the heat of adsorption of substances that are similar in shape and size of molecules, but do not form a hydrogen bond.
It is important to note that, knowing the thermodynamic description of the surface layer at the adsorbent-adsorbent interface, its structure, the nature of various types of forces, and the dynamics of the process, one can proceed to the study of more complex adsorption processes.
35. Adsorption as spontaneous concentration on the interface of substances that reduce interfacial tension
Surfactants are divided into two large groups: active and inactive substances.
Surfactants are able to accumulate in the surface layer, and positive adsorption occurs G > 0.
These types of substances must have a surface tension, which, in turn, must be less than the surface tension of the solvent, otherwise the accumulation of the substance in the surface layer will be unfavorable, and must have relatively low solubility. With sufficiently good solubility, surfactant molecules tend to move from the surface into the depths of the solution. Consequently, surfactants will be preferentially pushed out of the bulk of the liquid to the surface.
But with the accumulation of substances at the boundary of the solution in the molecules of these substances, which weakly interact with each other, the intermolecular interaction in the surface layer will decrease, and the surface tension will fall.
Surfactants relative to the aqueous layer are many types of organic compounds, fatty acids with a fairly large hydrocarbon radical, salts of these acids (soaps), sulfonic acids and their salts, as well as various types of alcohols and amines. A characteristic feature of most molecules is their diphilicity: the molecule consists of two parts of a polar group and a non-polar hydrocarbon radical. A polar group that has a significant dipole moment and is highly hydrating can determine the surfactant's affinity for the aqueous environment. But the hydrocarbon radical is the reason that reduces the solubility of these compounds.
Surface-inactive surfactants- these types of substances tend to leave the surface of the liquid into its volume, resulting in the so-called. negative adsorption G < 0. Поверностно-инактивные вещества также обладают значительным поверхностным натяжением, значительно большим, чем натяжение у растворителя (иначе эти вещества способны самопроизвольно накапливаться в поверхностном слое), также обладают высокой растворимостью, что способствует их стремлению уйти с поверхности жидкости в объем. Взаимодействие между молекулами поверхностно-инактивного вещества и растворителя всегда больше, чем взаимодействие между самими молекулами растворителя, поэтому они и стремятся перейти в объем раствора. Surface-inactive substances In relation to water there are many inorganic electrolytes: acids, alkalis, salts. Surfactant molecules do not have a hydrophobic part and can disintegrate in water into highly hydrating ions.
Examples Surfactants are also some organic compounds in which the non-polar part of the molecule is absent or very small. These substances include formic and aminoacetic acids.
In non-aqueous solvents, inorganic electrolytes can also increase surface tension, depending on the solvent.
For example, when sodium iodide is introduced into methanol, the surface tension greatly increases; for ethanol, the surface tension is approximately 2 times greater. The surface activity of substances can depend not only on the nature of the substance, but also on the properties of the solvent. If any solvent has a high surface tension, then that solute can exhibit significant surface activity.
36. Adsorption theories
Let us consider the most common adsorption theories that describe certain types of adsorption at the “solid-gas” or “solid-solution” interface.
The theory of monomolecular adsorption by I. Langmuir.
1. Adsorption is localized and is caused by forces close to chemical ones.
2. Adsorption occurs only on active centers - protrusions or depressions on the surface of the adsorbent, characterized by the presence of free valences. The active centers are considered independent and identical.
3. Each active center is able to interact with only one adsorbate molecule; Only one layer of adsorbed molecules can form on the surface.
4. The adsorption process is reversible and equilibrium; the adsorbed molecule is retained by the active site for some time, after which it is desorbed; After some time, dynamic equilibrium is established.
Maximum possible adsorption value G o is achieved provided that all active centers are occupied by adsorbate molecules. Monomolecular adsorption isotherm equation relating the magnitude of adsorption G with adsorbate concentration WITH, has the form:
Where b– a constant value for a given “adsorbent – adsorbate” pair (the ratio of the rate constants of desorption and adsorption), numerically equal to the adsorbate concentration at which half of the active centers are occupied.

The Langmuir adsorption isotherm graph is shown in Figure 2. The constant b determine graphically by drawing a tangent to the adsorption isotherm at the point WITH= 0. When describing the process of gas adsorption in the equation, the concentration can be replaced by a proportional value of the partial pressure. Theory of monomolecular adsorption I. Langmuir applicable to describe the processes of adsorption of gases and dissolved substances at low pressures (concentrations) of the adsorbate.
Polyani's theory of polymolecular adsorption describes s-shaped adsorption isotherms, the shape of which indicates the possible interaction of adsorbed molecules with the adsorbate.
1. Adsorption is caused by physical forces.
2. The surface of the adsorbent is homogeneous, there are no active centers; adsorption forces form a continuous force field near the surface of the adsorbent.
3. Adsorption forces act at a distance greater than the size of the adsorbate molecule, i.e., there is a certain adsorption volume at the surface of the adsorbent, which is filled with adsorbate molecules during adsorption.
4. The attraction of an adsorbate molecule by the surface of the adsorbent does not depend on the presence of other molecules in the adsorption volume, as a result of which polymolecular adsorption is possible.
5. Adsorption forces do not depend on temperature, and, therefore, the adsorption volume does not change with a change in temperature.
Freundlich equation. The surface of the adsorbent is heterogeneous, interaction occurs between the adsorbed particles, and the active centers are not completely independent of each other. G. Freundlich suggested that the number of moles of adsorbed gas or dissolved substance per unit mass of the adsorbent (the so-called specific adsorption X/m), must be proportional to the equilibrium pressure (for gas) or the equilibrium concentration (for substances adsorbed from solution) of the adsorbent, raised to a certain power, which is always less than unity:
x / m = aP n; x / m = aC n.
Exponents n and proportionality factor A determined experimentally.
37. Thermodynamics of the adsorption process. Gibbs adsorption equation
To study the phenomenon of adsorption at the “solution-gas” interface, it is necessary to establish a connection between the excess of the adsorbed substance in the layer on the surface ( G), surfactant concentration in solution ( With) and surface tension ( σ ) at the “solution – gas” phase boundary. It is more expedient to consider the phenomena from a thermodynamic point of view and relate the adsorption of a dissolved substance to a change in the free energy of the surface or its surface tension. This connection was made W. Gibbs V 1876, which was named "Gibbs adsorption equation":
G = – With / RT x dσ/dc.
You can still imagine Gibbs equation, based on thermodynamics, using isobaric-isothermal potential G, chemical potentials μ 1 And μ2, and also using n 1 And n 2 number of moles of components. Having analyzed it taking into account entropy S, volume V and pressure P, we can write the following equation:
dG=– SDT+VdP+σds+ μ 1 dn 1 + μ 2 dn 2.
Let us equate it to zero, and taking into account constant temperature and pressure, it simplifies into an equation of the form:
SD σ + n 1 d μ 1 + n 2 d μ 1 = 0.
Taking into account the fact that for dilute solutions the chemical potential of the second component is expressed as follows:
μ 2 = μ 2 0 +RT ln c,
and given that the temperature is constant
dμ 2 =RTdnc,
substituting this equation into
![]()
we obtain the desired Gibbs adsorption equation. Based on the equation, it can be seen that if surface tension σ increases with concentration With, then the concentration of the solute on the surface layer is less than in the bulk of the solution (so-called negative adsorption), and if the surface tension σ decreases with increasing concentration With, then the concentration in the layer is greater than in the volume (positive adsorption), and, finally, if σ does not depend on With, then the concentration of the substance in the layer on the surface and in the volume is the same. The Gibbs equation was derived using thermodynamics. It is difficult to practically verify this equation, which is due to the difficulty of determining the concentration of the dissolved substance in the surface layer. Experienced way B. McBen found that a very thin layer of liquid was cut off from the surface of the solution using the device. Further determination of all parameters of the Gibbs equation showed that the experimentally found adsorption values, within the experimental error, coincided with the values calculated using the Gibbs equation. Due to the homogeneity and smoothness of the surface of any liquid, the usual concepts of active centers are completely inapplicable when studying adsorption on its surface. At a critical temperature, the difference between the adjacent phases disappears, and surface tension, as a rule, becomes equal to zero. Adsorption of gases and vapors has such a wide practical application that in the literature, especially in technical literature, one can find this concept, which is applied only in relation to processes on the surface of solids.
This concept, like the most general laws of adsorption, like the Gibbs equation considered, is applicable to all phase boundaries. Using the Gibbs equation and all the provisions that follow from it, having determined the value of Γ, it is possible to construct an adsorption isotherm.
38. Features of adsorption on microporous materials. Polyany's potential theory. Adsorption potential
Polyani's theory considers non-localized physical adsorption, which is directly caused by van der Waals forces between the adsorbent and the adsorbate (this can be considered the first position). The second position of this theory is the idea of a force (or potential) field of the adsorbent, which extends over a considerable distance from the surface; the adsorption layer that appears in this field is polymolecular. If we consider the adsorption of gases, then the density of this layer decreases along a certain normal from the surface. If we consider vapor adsorption, then a liquid layer of a certain thickness is formed on the surface. The field in Polyani's theory is considered as a series of equipotential surfaces, each surface corresponds to a certain potential value ε , and each subsequent surface will be smaller than the previous one. Each such surface in space cuts out layers of a certain volume, designated as v i. The task of the Polyanyi theory is to find the transition from the usual coordinates of the isotherm ( x, p) to field parameters εi And v i, with further establishment of the connection between these basic parameters. The first part of the problem, which Polyany laid down, is quite complex, and in many cases cannot have definite solutions, but for the case of vapor adsorption, this part of the problem is solved in a first approximation very simply. For a liquid adsorption layer, the filled part of the volume will be equal to:
v i = x(M/d),
Where d– density of the substance in the liquid state.
In his theory, M. Polyani introduces another provision about the absence of the so-called. field screening during adsorption, value ε in this theory, space is a constant value (something like the gravitational potential) regardless of whether certain adsorbate molecules exist between a given point and a solid surface or whether all space is free. Polyani introduces the concept adsorption potential ε , which represents the isothermal work of compression of steam when transferring it from equilibrium pressure R in the bulk phase away from the surface into the region of the surface layer with saturated vapor pressure p 0 then the expression for determining the potential will look like:
ε = RT ln R 0 / R.
Using this equation, you can go from coordinates x, p to coordinates ε And v and obtain a curve, which is called “characteristic”. Polyani discovered in his experiments that such curves, constructed from the experimental data of the obtained isotherms, have the following property: they are invariant with respect to T, or, in other words, all curves of this type can fit on one curve ε −ε .
M. Polyany accepted this position as a postulate, i.e.:
This Polyani property is of great practical importance; it can construct a family of isotherms from one experimental adsorption isotherm.
Polanyi's theory does not provide an analytical expression for the isotherm or the potential-volume function, but it does allow one to calculate the coordinate for any given temperature if at least one isotherm is known. This result is very important for technological calculations, because for similar gases on the same adsorbent, the adsorption curves can be close to each other and can be combined in many cases.
39. Characteristic adsorption curve. Temperature invariance and affinity of characteristic curves
The force field that arises at the surface of the adsorbent can be in many ways similar to the gravitational field. In the adsorption field, one can imagine potential surfaces, i.e., surfaces that are characterized by the same adsorption potential. Under the concept of adsorption potential θ should be understood as nothing more than the work done against adsorption forces when moving 1 mole of adsorbent from a certain point in the field to a certain gas phase. The maximum adsorption potential will exist at the “adsorbent – adsorption volume” boundary. But at the “volume – gas phase” boundary (this is where the action of adsorption forces ends), the adsorption potential should be equal to zero. The change in adsorption potential with a change in the adsorption volume can be represented in the form of curves. This was done for the first time by M. Polyani. These types of curves do not depend on temperature and can be characteristic of each specific adsorbent; these types of curves are usually called characteristic adsorption curves. The theory of polymolecular adsorption assumes that the gas equation of state is applicable for the adsorption volume. Consequently, the isotherms that characterize the dependence of the density of the adsorbent on the volume for different temperatures resemble the isotherms of the dependence of pressure on volume. At low temperatures, surface adsorption forces can cause vapor to condense into a liquid of a certain density. At temperatures lower than critical, during condensation, the entire adsorption volume will be filled with liquid. In this case, the adsorption curve will run almost parallel to the abscissa axis, which is associated with the low compressibility of the liquid. Then the adsorption curve at the “volume – gas phase” boundary sharply drops down, and, accordingly, the density of the adsorbent reaches a certain density of the gas phase. At temperatures higher than the critical temperature, the adsorptive can behave like an ideal gas, and the graph will be expressed as an ideal gas isotherm, provided that pV = RT. Under such conditions, the adsorbed gas will have a maximum density at the very surface of the adsorbent and a minimum density in the immediate vicinity of the gas phase. Moreover, in this case, it is important to note that the density of the adsorbent in the adsorption layer nowhere reaches the density of the liquid itself. And if the temperature is very close to critical, the dependence of density on volume will be expressed by a curve close in appearance to the isotherm, which is described van der Waals equation. In this situation, part of the adsorbed substance will be in the adsorbed volume in a liquid state, and part of the adsorbed substance will be in a gaseous state. Then the curve will decrease most sharply in the section that corresponds to the transition from liquid to gas. If you construct a characteristic curve from the experimental adsorption isotherm of one of the adsorptives, and knowing the corresponding affinity coefficients for some other adsorptive, you can find the adsorption isotherm and construct it for another adsorptive. The potential theory of adsorption makes it possible to calculate different adsorption isotherms of different vapors on the same adsorbent, using a characteristic curve that is obtained from the adsorption isotherm of one vapor, since the ratio of the adsorption potential does not depend on the adsorption volumes.
Affinity(from Latin affinis - “related”) - affinity chromatography. The method for purifying and separating proteins is based on their selective interaction with a ligand covalently bound to an inert carrier (affinity chromatography). Measuring the affinity of a toxicant for a receptor is essentially an experimental study of the relationship between the amount of a substance added to the incubation medium and the amount of the toxicant-receptor complex formed as a result of interaction.
Thermodynamics of adsorption processes.
| Parameter name | Meaning |
| Article topic: | Thermodynamics of adsorption processes. |
| Rubric (thematic category) | Education |
Basic definitions and methods of classification of adsorption processes.
Adsorption refers to phenomena that occur due to a spontaneous decrease in surface energy.
Adsorption– the process of spontaneous reversible or irreversible redistribution of the components of a heterogeneous system between the surface layer and the volume of the homogeneous phase.
In multicomponent systems, the component that more strongly reduces the interfacial tension is preferably transferred to the surface layer. In one-component systems, during the formation of the surface layer, a change in its structure occurs (a certain orientation of atoms and molecules, polarization), called autoadsorption.
The denser phase on which adsorption interactions are localized is called adsorbent. The substance redistributed between the volume of the homogeneous phase and the surface layer is designated by the term ʼʼ adsorbateʼʼ.
In some cases, the adsorption process is reversible. In this case, under certain conditions, part of the adsorbed molecules as a result of molecular kinetic phenomena can move from the surface layer to the bulk phase. The reverse process of adsorption is called desorption.
Methods for classifying adsorption processes.
Classification of adsorption processes according to the state of aggregation of interacting phases. Taking into account the dependence on the aggregate state of adjacent phases, the following types of adsorption processes are distinguished:
Adsorption of gases on solid adsorbents;
Adsorption of dissolved substances at the “solid-liquid” and “liquid-liquid” interfaces;
Adsorption of surfactants at the liquid-gas interface.
Classification of adsorption processes according to the mechanism of interaction between the adsorbent and the adsorbate. Adsorption can be considered as the interaction of adsorbate molecules with the active centers of the adsorbent. According to the mechanism of their interaction, the following types of adsorption are divided:
1) physical (molecular) adsorption– the interaction between the molecules of the adsorbate and the adsorbent is carried out due to van der Waals forces, hydrogen bonds (without chemical reactions);
2) chemical adsorption (chemisorption)– the attachment of adsorbate molecules to the active centers of the adsorbent occurs as a result of chemical reactions of various types (with the exception of ion exchange reactions);
3) ion exchange adsorption (ion exchange) – redistribution of the adsorbate substance between the solution and the solid phase (ion exchanger) according to the mechanism of ion exchange reactions.
To quantitatively describe adsorption processes, two quantities are used.
1) Absolute adsorption– quantity (mol) or mass (kg) of adsorbate per unit surface area or mass of the adsorbent. Designation – A; dimension: mol/m2, mol/kg, kg/m2, kg/kᴦ.
2) Gibbs (excess) adsorption– excess of adsorbate substance in a surface layer of a certain thickness compared to its amount in the volume of the homogeneous phase, per unit surface area or mass of the adsorbent. Designation – G; dimension: mol/m 2, mol/kᴦ.
The relationship between absolute and excess adsorption can be illustrated using the equation:
Г = А – с * h (3.1)
where c is the equilibrium concentration of the substance in the volume of the phase, mol/m3;
h is the thickness of the surface layer, conventionally assumed to be 10 -9 m.
In multicomponent heterogeneous systems, when one or another component is redistributed between the volume of the homogeneous phase and the surface layer, the equation for the excess internal energy of the surface is valid:
U = T * S + s * s + Sm i * n i (3.2)
Reducing all terms of the equation to unit area of the interphase surface, we obtain:
U s = T * S s + s + Sm i * Г i (3.3)
where Г i = n i / s is the excess of the i-th component in the surface layer, that is, Gibbs adsorption.
For a one-component system, equation (3.3) will take the form:
G s = s + m * G (3.4)
where G s = U s - T * S s – surface Gibbs energy or work of creation per unit surface area;
m * G – compaction of the substance of the adsorbed substance in the surface layer.
Based on equation (3.4), we can conclude that during adsorption, the work of creating an interphase surface consists of the work of surface formation (breaking cohesive bonds in the volume of the adsorbate phase) and compaction of the substance in the surface layer.
In a state of dynamic equilibrium between the adsorbent and the adsorbate, the change in the Gibbs energy of the heterogeneous system ΔG = 0, the thermodynamics of the adsorption process is described by the equation called Gibbs fundamental adsorption equation:
Ds = SГ i * dm i (3.5)
This equation is universal, as it is valid for all types of adsorption processes
Special cases of the Gibbs adsorption equation.
1) Adsorption from solutions.
For the chemical potential of the ith component of the system during adsorption at the “liquid – solid adsorbent” and “liquid – gas” interfaces, the following equations are valid:
m i = m i 0 + R*T*ln a i (3.6)
dm i = R*T* d ln a i (3.7)
where m i 0 is the chemical potential of the i-th component of the system under standard conditions;
a i is the activity of the i-th component of the system under standard conditions.
Based on this, the Gibbs adsorption equation takes the form:
Г i = - a i / R*T * (ds / da i) (3.8)
For solutions of non-electrolytes we take a i = c i, then:
Г i = - с / R*T * (ds / dс) (3.9)
For electrolyte solutions:
Г i = - с ± n / R*T * (ds / dс ± n) (3.10)
where с ± is the average ionic concentration of the solution;
n is the stoichiometric coefficient.
2) Adsorption of substances from the gas phase.
In accordance with the Mendeleev-Clayperon equation:
Р = с * R*T (3.11)
In this regard, the Gibbs equation for the adsorption of gases on solid adsorbents is written in the following form:
Г i = - Р / R*T * (ds / dР) (3.12)
In practice, the Gibbs adsorption equation allows, based on surface tension measurements at various values of liquid concentration or equilibrium gas pressure, to calculate the amount of adsorption of substances in the interfacial layer for which the surface tension is determined.
Thermodynamics of adsorption processes. - concept and types. Classification and features of the category "Thermodynamics of adsorption processes." 2017, 2018.