Types of chemical bonds in organic compounds. The structure of organic compounds and electronic effects What types of bonds occur between carbon atoms
Chemical bond - these are the forces of interaction between atoms or groups of atoms, leading to the formation of molecules, ions, etc. By its nature, a chemical bond is electrostatic forces. The main role in the formation of chemical bonds between atoms is played by them valence electrons, i.e. electrons of the outer level, least tightly bound to the nucleus. From the general chemistry course you know about the existence of covalent and ionic bonds.
Ionic bond is a chemical bond based on the electrostatic attraction of ions. It occurs when there is a large difference in the electronegativities of the bonded atoms. In organic compounds, ionic bonds are quite rare, for example, in salts of carboxylic acids:
For organic compounds, covalent bonds are most characteristic.
Covalent bond is a chemical bond formed by sharing electrons between two or more nuclei. There are two ways to share an electron pair: exchange and donor-acceptor.
By exchange In the mechanism of bond formation, one electron from each atom is involved:
N · + Cl · → H : Cl
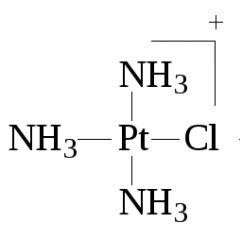
By donor-acceptor According to the mechanism, a covalent bond is formed by combining an electron pair of one atom and a free orbital of another. An example is the interaction of an amine molecule with a proton to form a methylammonium cation:

Classification of covalent bonds according to the methods of overlapping atomic orbitals
Depending on the method of overlapping atomic orbitals, σ- and π-bonds are distinguished. σ-bonds are formed as a result of overlapping orbitals along a line connecting the centers of the nuclei of two atoms:

π -communications are formed as a result of lateral p-p-overlapping of orbitals, as a result of which two regions of increased electron density are formed:

Types of covalent bond cleavage
The cleavage of a covalent bond can occur by homolytic or heterolytic mechanisms.
Homolytic reactions- reactions in which bond cleavage occurs symmetrically, so that each of the resulting fragments loses one electron:
Homolysis from Greek homos- identical,lysis- gap.
During homolytic reactions, free radicals are formed as intermediates - particles containing an unpaired electron, for example:

Radical- an atom or group of atoms that has an unpaired electron.
Heterolytic reactions- reactions in which bond cleavage occurs asymmetrically, so that a pair of bond electrons remains with one of the resulting fragments:
Heterolysis - This is an asymmetric rupture of a covalent bond, as a result of which particles of different natures are formed: a cation and an anion.
If the charges in such particles are on the carbon atom, they are called carbocations and carbanions, for example:
The electron pair remains with the more electronegative atom.
Carbon atoms in organic compounds are tetravalent and can be in three different states of hybridization (Table 22.1).
Table 22.1
Hybridization of carbon atoms
In the formation of organic compounds, a special role is played by the ability of carbon atoms to connect with each other to form chains, branched chains and cycles. C-C bonds are significantly stronger than bonds between other identical atoms, which explains the stability of carbon structures:
The interconnected carbon atoms are called the carbon skeleton of the molecule.
The spatial configuration of carbon structures is determined by the hybridization of carbon atoms. When ^-hybridization of all atoms occurs, zigzag chains are formed. When a cycle is formed, the carbon atoms deviate from their planar arrangement. Examples are presented in the diagrams:

If the zigzag chain contains carbon atoms in the 5p state 2-hybridization, then areas with a planar arrangement of atoms appear. In the presence of carbon atoms in the state of sp-hybridization, linear sections of the chain appear.
The terminal carbon atoms, called primary, have three valences left for the addition of other atoms and atomic groups: H, OH, Cl, NH 2 etc. Non-terminal atoms bonded to two carbon atoms are calledsecondary. Two more atoms are added to them. There are no primary carbon atoms in the carbon cycle. If there is a branch in the chain, it appears tertiary carbon atom. It has only one valency left for the addition of other atoms. Finally, in a chain of carbon atoms two branches can arise from one atom. Such an atom is called quaternary; it is bonded only to carbon atoms:

In a molecule with one carbon atom, this atom is calledisolated.
Depending on the type of hybridization, carbon forms single (a) and multiple - double (a + l) and triple (a + 2l) bonds. l-Bonds can occur not only between carbon atoms, but also with atoms attached to carbon. A special type of chemical bond is conjugate a double bond that occurs when there are more than two atoms in the carbon chain in the state of 5p 2 hybridization (see Fig. 6.26). It follows from the figure that unpaired electrons in non-hybrid p-orbitals can form bonds between any adjacent carbon atoms, and this leads to delocalization of the p-bond along the entire chain of $p 2 carbon atoms. In chemical reactions, the presence of an n-bond can appear between atoms 1 And 2, then between atoms 2 and 3, etc.
Compounds in which there are multiple bonds and, accordingly,sp 2 -and sp-carbon atoms are calledunsaturated. If these are hydrocarbons, then the hydrogen content in them is less than the maximum possible. These compounds exhibit increased reactivity, since the electron cloud of the n-bond is concentrated on two sides of the C atoms and therefore quite easily moves from one of the two atoms to the other under the influence of reagent molecules.
The most important classes of organic compounds, in addition to carbon and hydrogen, may contain oxygen, nitrogen, halogens, and sulfur. Of these elements, hydrogen has a lower electronegativity than carbon, while the others have a higher electronegativity. Covalent bonds of carbon with them are more or less polar, and the atoms have partial electrical charges ± 8 :
The polarity of bonds affects the reactivity of compounds.
Carbon atoms have the inherent ability to form stable bonds with several different atoms at once. This leads to many combinations rarely encountered in inorganic chemistry. Let's compare carbon and aluminum. The latter forms four halides (AIF3, A1C1 3, A1Br 3, AP 3) and the hydride A1H 3. Carbon can produce many molecules with the simultaneous presence of different halogens, as well as hydrogen and other carbon atoms: CH 3 C1, CH 2 C1 2, CH 2 ClBr, CHFClBr, CH 3 CHC1Br, etc. This is also one of the reasons for the diversity of organic compounds.
In organic chemistry, structural formulas of molecules are widely used. Structural formulas can be depicted with varying degrees of specificity and approximation to the real structure. Let's consider several types of formulas depicting the propane molecule.

In polyatomic molecules of organic compounds, continuous rotation of atomic groups around axes coinciding with the direction of single C-C bonds is possible (for short, they say: rotation around a C-C bond). In the simplest case of ethane C 2 H 6, two CH 3 groups rotate almost freely relative to each other, like two wheels loosely mounted on an axle:

Molecules with carbon chains of four or more atoms bend like a caterpillar during internal rotation, creating all sorts of conformation(mutual positions) of atoms both in volume and on the plane. A chain of five carbon atoms has three planar conformations:

Between the three planar conformations, three-dimensional transition conformations arise. The horseshoe conformation is favorable for the formation of a cyclic structure.
Organic molecules have individual parts (fragments) that differ in composition. Attached to the main carbon chain or ring may be branches consisting of carbon and hydrogen, called hydrocarbon radicals. The simplest radicals already encountered in the text are methyl -CH 3 and ethyl -C 2 H 5 . The fourth bond of the radical is represented by a dash or dot (CH 3). The remaining elements, except carbon and hydrogen, in molecules of organic compounds are considered as functional groups. This term is due to the fact that chemical reactions occur predominantly with the participation of these groups. In the organic compounds CH 3 COOP and C 2 H 5 NH 2 already encountered in the text, there are functional groups -COOH (carboxyl) with acidic properties and -NH 2 (amino group) with basic properties.
The reactivity of organic compounds is determined by the type of chemical bonds and the mutual influence of atoms in the molecule. These factors, in turn, are determined by the interaction of atomic orbitals (AO).
The part of space in which the probability of finding an electron is maximum is called an atomic orbital.
In organic chemistry, the concept of hybrid orbitals of the carbon atom and other elements is widely used. The concept of orbital hybridization is necessary in cases where the number of unpaired electrons in the ground state of an atom is less than the number of bonds it forms. It is postulated that different atomic orbitals of similar energy interact with each other to form hybrid orbitals of the same energy. Hybrid orbitals, due to their large overlap, provide a stronger bond than non-hybridized orbitals. Depending on the number of orbitals that have entered into hybridization, a carbon atom can be in three types of hybridization:
1. First valence state, sp3 hybridization (tetrahedral)
As a result of a linear combination (mixing) of four AOs of an excited carbon atom (one 2s and three 2p), four equivalent sp 3 hybrid orbitals arise, directed in space to the vertices of the tetrahedron at angles of 109.5?. The shape of the hybrid orbital is a three-dimensional figure eight, one of the blades of which is much larger than the other.
2. Second valence state, sp2 - hybridization (triangular)
It arises as a result of the displacement of one 2s and two 2p atomic orbitals. The resulting three sp 2 hybrid orbitals are located in the same plane at an angle of 120? to each other, and non-hybridized p - AO - in a plane perpendicular to it. In the state of sp 2 hybridization, the carbon atom is found in alkene molecules, carbonyl and carboxyl groups
3. Third valence state, sp - hybridization
It arises as a result of mixing one 2s and one 2p AO. The resulting two sp hybrid orbitals are located linearly, and the two p orbitals are located in two mutually perpendicular planes. The carbon atom in the sp hybrid state is found in molecules of alkynes and nitriles
There are three possible types of bonds connecting individual atoms of elements in a compound - electrostatic, covalent and metallic.
Electrostatic bonds primarily include ionic bonds, which occur when one atom transfers an electron or electrons to another, and the resulting ions are attracted to each other.
Organic compounds are characterized mainly by covalent bonds. A covalent bond is a chemical bond formed by sharing the electrons of the bonded atoms.
For the quantum mechanical description of covalent bonds, two main approaches are used: the valence bond (VB) method and the molecular orbital (MO) method. chemical covalent molecule
The BC method is based on the idea of electron pairing that occurs when atomic orbitals overlap. A generalized pair of electrons with opposite spins forms a region with increased electron density between the nuclei of two atoms, attracting both nuclei. A two-electron covalent bond occurs. According to the BC method, atomic orbitals retain their individuality. Therefore, both paired electrons remain in the atomic orbitals of the bonded atoms, i.e., they are localized between the nuclei.
In the initial stage of development of electronic theory (Lewis), the idea of a covalent bond as a socialized pair of electrons was put forward. To explain the properties of different atoms to form a certain number of covalent bonds, the octet rule was formulated. According to him, during the formation of molecules from atoms of the 2nd period of the periodic system, D.I. Mendeleev, the outer shell is filled with the formation of a stable 8-electron system (shell of an inert gas). Four electron pairs can form covalent bonds or exist as lone pairs.
When moving to the elements of the third and subsequent periods, the rule of the octet loses its force, since d-orbitals appear that are quite low in energy. Therefore, atoms of higher periods can form more than four covalent bonds. Lewis's assumptions about a chemical bond as a social pair of electrons were of a purely qualitative nature.
According to the MO method, bond electrons are not localized on AOs of certain atoms, but are located on MOs, which are a linear combination of atomic orbitals (LCAO) of all atoms that make up the molecule. The number of formed MOs is equal to the number of overlapping AOs. A molecular orbital is usually a multicenter orbital and the electrons that fill it are delocalized. The filling of MOs with electrons occurs in compliance with the Pauli principle. A MO obtained by adding the wave functions of atomic orbitals and having a lower energy than the AOs that form it is called bonding. The presence of electrons in this orbital reduces the overall energy of the molecule and ensures the bonding of atoms. A high-energy MO obtained by subtracting wave functions is called antibonding (antibonding). For an antibonding orbital, the probability of finding electrons between nuclei is zero. This orbital is vacant.
In addition to binding and antibonding MOs, there are also non-binding MOs, designated n-MOs. They are formed with the participation of AOs carrying a pair of electrons that are not involved in the formation of the bond. Such electrons are also called free lone pairs or n-electrons (they are found on nitrogen, oxygen, and halogen atoms).
There are two types of covalent bonds: y- (sigma) and p- (pi) bonds.
A y-bond is a bond formed by the axial overlap of any (s-, p- or hybrid sp- atomic orbitals) with the maximum overlap located on the straight line connecting the nuclei of the bonded atoms.
According to the MO method, y-overlap leads to the appearance of two MOs: a bonding y-MO and an antibonding y*-MO.
A p-Bond is a bond formed by lateral (lateral) overlap of a p-AO, with the maximum electron density located on both sides of the straight line connecting the nuclei of atoms. According to the MO method, as a result of a linear combination of two p-AOs, a binding p-MO and an antibonding p*-MO are formed.
A double bond is a combination of y- and p-bonds, and a triple bond is one y- and two p-bonds.
The main characteristics of a covalent bond are energy, length, polarity, polarizability, directionality and saturability.
Bond energy is the amount of energy released during the formation of a given bond or required to separate two bonded atoms. The greater the energy, the stronger the connection.
Bond length is the distance between the centers of bonded atoms. A double bond is shorter than a single bond, and a triple bond is shorter than a double bond.
Bond polarity is determined by the uneven distribution (polarization) of electron density, the reason for which is the difference in electronegativity of bonded atoms. As the difference in electronegativity between bonded atoms increases, the polarity of the bond increases. Thus, one can imagine the transition from a non-polar covalent bond through a polar to an ionic bond. Polar covalent bonds are prone to heterolytic cleavage.
Bond polarizability is a measure of the displacement of bond electrons under the influence of an external electric field, including that of another reacting particle. Polarizability is determined by electron mobility. Electrons are more mobile the further they are from the nuclei.
In organogens (carbon, nitrogen, oxygen, sulfur, halogens) in the formation of y-bonds, the participation of hybrid orbitals is energetically more favorable, providing more efficient overlap.
The overlap of two one-electron AOs is not the only way to form a covalent bond. A covalent bond can be formed by the interaction of a filled two-electron orbital (donor) with a vacant orbital (acceptor). Donors are compounds containing either orbitals with a lone pair of electrons or p - MO. A covalent bond formed by an electron pair of one atom is called donor-acceptor or coordination.
A type of donor-acceptor bond is a semipolar bond. For example, in a nitro group, simultaneously with the formation of a covalent bond due to the lone pair of nitrogen electrons, charges of opposite sign appear on the bonded atoms. Due to electrostatic attraction, an ionic bond occurs between them. The resulting combination of covalent and ionic bonding is called a semipolar bond. The donor-acceptor bond is characteristic of complex compounds. Depending on the type of donor, n- or p-complexes are distinguished.
A hydrogen atom bonded to a strongly electronegative atom (N, O, F) is electron-deficient and is capable of interacting with the lone pair of electrons of another strongly electronegative atom, located either in the same or in another molecule. As a result, a hydrogen bond occurs. Graphically, a hydrogen bond is indicated by three dots.
The hydrogen bond energy is low (10-40 kJ/mol) and is mainly determined by electrostatic interaction.
Intermolecular hydrogen bonds cause the association of organic compounds, which leads to an increase in the boiling point of alcohols (t? boiling point C 2 H 5 OH = 78.3? C; t? boiling point CH 3 OCH 3 = -24? C), carboxylic acids and many other physical (t? melting point, viscosity) and chemical (acid-base) properties.
Intramolecular hydrogen bonds can also occur, for example in salicylic acid, which leads to an increase in its acidity.
The ethylene molecule is flat, the angle between the H - C - H bond is 120? C. In order to break the p - p - double bond and make it possible to rotate around the remaining sp 2 - y - bond, it is necessary to expend a significant amount of energy; Therefore, rotation around the double bond is difficult and the existence of cis- and trans-isomers is possible.
A covalent bond is nonpolar only when bonding atoms that are identical or similar in electronegativity. When electrons bond, the covalent bond density shifts toward the more electronegative atom. This relationship is polarized. Polarization is not limited to just one y-bond, but spreads along the chain and leads to the appearance of partial charges (y) on the atoms
Thus, the “X” substituent causes polarization not only of its y-bond with the carbon atom, but also transmits its influence (exhibits an effect) to neighboring y-bonds. This type of electronic influence is called inductive and is denoted j.
The inductive effect is the transfer of the electronic influence of a substituent along a chain of y-bonds.
The direction of the inductive effect of a substituent is usually assessed qualitatively by comparison with the hydrogen atom, the inductive effect of which is taken to be 0 (the C-H bond is considered practically non-polar).
Substituent X, which attracts the electron density of the y-bond more strongly than the hydrogen atom, exhibits a negative inductive effect -I. If, compared to the hydrogen atom, the substituent Y increases the electron density in the chain, then it exhibits a positive inductive effect, +I. Graphically, the inductive effect is represented by an arrow coinciding with the position of the valence line and pointing towards the more electronegative atom. The +I effect is exerted by alkyl groups, metal atoms, and anions. Most substituents have an -I effect. And the greater, the higher the electronegativity of the atom forming a covalent bond with the carbon atom. Unsaturated groups (all without exception) have an -I effect, the magnitude of which increases with increasing multiple bonds.
The inductive effect, due to the weak polarizability of the y-bond, decays after three or four y-bonds in the circuit. Its effect is strongest on the first two carbon atoms closest to the substituent.
If a molecule contains conjugated double or triple bonds, the conjugation effect (or mesomeric effect; M-effect) occurs.
The conjugation effect is the transfer of the electronic influence of a substituent through a system of p-bonds. Substituents that increase the electron density in a conjugated system exhibit a positive conjugation effect, the +M effect. The +M effect is exhibited by substituents containing atoms with a lone pair of electrons or a whole negative charge. Substituents that withdraw electron density from the conjugated system exhibit a negative (mesomeric) conjugation effect, the -M effect. These include unsaturated groups and positively charged atoms. The redistribution (displacement) of the total electron cloud under the influence of the M effect is graphically depicted by curved arrows, the beginning of which shows which p- or p-electrons are displaced, and the end - the bond or atom to which they are displaced
The mesomeric effect (conjugation effect) is transmitted through a system of conjugated bonds to significantly greater spreads.
A covalent bond can be polarized and delocalized.
Localized covalent bond - the bonding electrons are shared between the two nuclei of the atoms being bonded.
A delocalized bond is a covalent bond whose molecular orbital spans more than 2 atoms. These are almost always p-connections.
Conjugation (mesomerism, mesos - average) is the phenomenon of alignment of bonds and charges in a real molecule (particle) in comparison with a real, but non-existent structure.
Resonance theory - a real molecule or particle is described by a set of specific, so-called resonance structures, which differ from each other only in the distribution of electron density.
Organic chemistry is of exceptionally important educational and economic importance.
Natural organic substances and their transformations underlie the phenomena of Life. Therefore, organic chemistry is the chemical foundation of biological chemistry and molecular biology - sciences that study the processes occurring in the cells of organisms at the molecular level. Research in this area allows us to better understand the essence of living natural phenomena.
Many synthetic organic compounds are produced by industry for use in a variety of sectors of human activity - these are petroleum products, fuel for various engines, polymeric materials (rubbers, plastics, fibers, films, varnishes, adhesives, etc.), surfactants, dyes , plant protection products, medicines, flavoring and perfumery substances, etc. Without knowledge of the basics of organic chemistry, modern man is not able to competently use all these products of civilization.
The raw materials for organic compounds are oil and natural gas, coal and brown coal, oil shale, peat, agricultural and forestry products.
The criterion for dividing compounds into organic and inorganic is their elemental composition.
Organic compounds include chemicals containing carbon, for example:
CH 3 -CN, CH 3 -CH 2 -OH, CS 2, CH 3 COOH, CH 3 -NH 2, CH 3 -NO 2, CH 3 -COOC 2 H 5 .
Organic compounds differ from inorganic compounds in a number of characteristic features:
· almost all organic substances burn or are easily destroyed when heated with oxidizing agents, releasing CO 2 (based on this feature, it is possible to determine whether the substance under study is an organic compound);
· in molecules of organic compounds, carbon can be combined with almost any element of the periodic table;
Organic molecules may contain a sequence of carbon atoms connected in chains (open or closed);
· molecules of most organic compounds do not dissociate into sufficiently stable ions;
· reactions of organic compounds proceed much more slowly and in most cases do not reach completion;
Widespread phenomenon among organic compounds isomerism ;
· organic substances have lower phase transition temperatures (bp, mp).
There are much more organic compounds than inorganic ones.
Basic principles of Butlerov's theory of chemical structure
1. Atoms in molecules are connected to each other in a certain sequence according to their valencies. The sequence of interatomic bonds in a molecule is called its chemical structure and is reflected by one structural formula (structure formula).
2. The chemical structure can be determined using chemical methods. (Modern physical methods are also currently used).
3. The properties of substances depend on their chemical structure.
4. Based on the properties of a given substance, one can determine the structure of its molecule, and based on the structure of the molecule, one can predict the properties.
5. Atoms and groups of atoms in a molecule have a mutual influence on each other.
From the moment when researchers learned to determine the elemental composition of compounds, it was noticed that often compounds with the same elemental composition have completely different chemical and physical properties. Identification of the reasons for this behavior stimulated the creation of a theory of the structure of organic compounds. This theory was first formulated by A.M. Butlerov.
Butlerov's theory was the scientific foundation of organic chemistry and contributed to its rapid development. Based on the provisions of the theory, A.M. Butlerov gave an explanation for the phenomenon isomerism , predicted the existence of various isomers and obtained some of them for the first time.
Structure of the carbon atom
It is obvious that all reactions in which organic molecules enter are associated with the structure of the carbon atom of a particular molecule and the rearrangement of its outer valence orbitals during the transformation process.
In an unexcited state, a carbon atom has 2 electrons in the s orbital of the second sublevel (2s orbitals), as well as 2 electrons in two (out of a total of 3) p orbitals of the 2nd sublevel (2p x and 2p y orbitals):
Thus, carbon has 4 electrons in its outer orbitals that are capable of forming bonds. According to the theory, the shapes of the s and p orbitals describe the probability of finding an electron relative to the nucleus of an atom. Non-hybridized s- and p-orbitals have the shape of a sphere and a uniform “dumbbell” and are located in space according to the diagram below:
When compounds are formed from atomic carbon (or as part of carbon compounds), a change occurs in the shape and location in space relative to the nucleus of the atom of the outer orbitals of carbon, called hybridization . Hybridization can be schematically represented as follows:

Out of four unhybridized atomic s- and p-orbitals having different shapes, as a result sp 3 hybridization (which means changing one s- and three R-orbitals) are obtained four equal by energy and form hybridized e molecular orbitals shaped like distorted dumbbells.
To ensure minimal steric hindrance and mutual repulsion, these four equivalent orbitals are located in space at equal distances from each other, directed towards the vertices of the tetrahedron (the nucleus of the carbon atom is located in the center of the tetrahedron), and the spatial angles between the orbitals are about 109° 28':

In this state, four bonds as a result of overlapping orbitals can be formed without hindrance. In such hybridization, carbon is present (exclusively) in the composition of alkanes, cycloalkanes and alcohols.
Thus, for example, an ethane molecule looks like (yellow spheres show hydrogen atoms, more precisely, their s orbitals):

The bond between carbon atoms is formed by overlapping hybridized orbitals. Such connections are called s- bonds (sigma bonds). Around s- connection, rotation of molecular fragments is possible.
Hybridization is a change in the shape and location in space relative to the nucleus of an atom of its outer electron orbitals, when forming bonds with other atoms. Another definition: hybridization – orbital mixing , as a result of which they align in shape and energy.
A carbon atom that has a multiple bond (alkenes -C=C -, carbonyl compounds >C =O, carboxylic acids and their derivatives -COOH, -COOR, etc.) has a different hybridization (sp 2), respectively, shape and location in space of outer orbitals:

In the state of sp 2 hybridization at carbon, there are only 3 hybridized orbitals (derived from one s and two p orbitals), which are located in the same plane at an angle of 120° between them, and the fourth ( unhybridized) the p-orbital is located perpendicular to this plane. A double bond is formed as a result overlap of unhybridized orbitals between neighboring carbon atoms (or between carbon and oxygen), the figure shows an ethylene (ethene) molecule:

Bonds formed by overlapping unhybridized p-orbitals are called p- connections. Thus, a multiple (double) bond in an ethene molecule is formed by one sigma and one pi bond.
Rotation of molecular fragments around p- bonding is, for obvious reasons, impossible at normal temperature (additional energy is required to break the overlapping p-orbitals), this determines the presence of spatial (geometric) isomers in alkenes, subject to some additional conditions, which will be discussed below.
On the image unhybridized p-orbitals are located at a distance - artificially spaced apart for better perception, although in reality they “touch” each other, overlapping above and below, but forming only one additional bond.
The carbon at the triple bond (in alkynes and nitriles) is in the state sp hybridization :

A pair of hybridized orbitals are located in a line, at an angle of 180° and in opposite directions. The two unhybridized p-orbitals, according to the principle of minimum repulsion and to minimize steric hindrance, are located perpendicular to this line and at an angle of 90° to each other. The triple bond in alkynes is formed as a result of overlapping hybridized orbitals (one s- bond) and two unhybridized p-orbitals of neighboring carbon atoms (two p-bonds). For example, this is what the model of the acetylene molecule (ethyn) looks like:

As a result of reactions, carbon is able to both change and maintain its hybridization state.
Types of bonds in molecules of organic substances
The predominant type of bond in molecules of organic compounds is a covalent bond. A pair of bonding electrons is shared approximately equally between atoms when characterizing C-C or C-H bonds. This is caused by approximately equal electron affinities ( electronegativity) C and H atoms.
In the case where carbon is bonded to a more electronegative atom (halogens, oxygen, nitrogen), the bond can be significantly polarized, and partial positive (on carbon) and negative (on halogen, oxygen, nitrogen) charges can form on the atoms. However, the degree of ionicity of such a bond is minimal.
Due to the non-polarity of the C-C and C-H bonds, the preferred method of breaking it is homolytic, when a pair of electrons is shared equally between the atoms. When a bond is broken this way, uncharged but highly reactive particles with unpaired electrons, called radicals, are formed. Reactions with the intermediate formation of radicals are very typical for alkanes. Such transformations are initiated by introducing from the outside energy sufficient to break the bond (heating) or compounds that initiate the formation of radicals with weak heating or ultraviolet irradiation (peroxides, halogens, azo compounds, chemical initiators that generate radicals as a result of a chemical reaction). In general, alkanes and cycloalkanes with unstrained rings are chemically relatively inert.
In contrast, alkenes are much more reactive. The reason for this is the unsaturation (multiple bond) and availability of the loose electron density of the overlapping p orbitals p- bonds for the action of electrophilic reagents (compounds with empty outer orbitals or electron deficient connections). As a result, the multiple bond disappears and electrophiles join. The reactions proceed with the intermediate formation of positively charged intermediates (carbocations) or radicals.
Another group of reactions is associated with the polarization of the carbon-halogen, oxygen or nitrogen bond. These reactions have a more complex mechanism and depend on the structure of the substrate, reagent and reaction conditions (solvent, catalyst, etc.).
There are also more complex types of reactions ( cycloaddition or Diels–Alder reaction), the detailed mechanism of which has not yet been studied in all its intricacies.
Types of reactions in organic chemistry
Thus, it is possible to distinguish only a few types of reactions in which organic compounds enter:
1) reactions substitution when one atom (or group of atoms) is replaced by another atom (or group of atoms). The carbon skeleton remains unchanged. Reactions proceed through a preliminary cleavage of the bond followed by the formation of a new one;
2) reactions accession . Characteristic of compounds that have unsaturation (multiple bonds), as a result of which the addition of other molecules (hydrogen, water, halogens, oxygen, hydrogen halides, etc.) is possible;
3) reactions splitting off (elimination), when molecules (water, ammonia, halogens, hydrogen halides, hydrogen, CO, CO 2, etc.) are split off from a molecule of an organic compound. Such reactions are often named after the type of molecule being removed, respectively, dehydration, deamination, dehalogenation, dehydrohalogenation, dehydrogenation, decarbonylation, decarboxylation etc.;
4) reactions condensation when the carbon skeleton of a molecule becomes enlarged;
5) cracking (or splitting) reactions, as a result of which the carbon skeleton is split into smaller molecules;
6) reactions oxidation , accompanied by the removal of hydrogen molecules (a special case of an elimination reaction), or with the simultaneous introduction of oxygen molecules (conversion of alcohols into aldehydes and ketones and, further, into acids);
7)reactions isomerization (or restructuring of the carbon skeleton or cycles);
8)reactions polymerization , as a result of which long, unbranched polymer molecules are obtained from small molecules (monomers). In living nature, there are examples of the formation of branched polymer molecules, the structural units of which are organic molecules of monosaccharides (carbohydrates).
Classification of organic compounds
Despite the variety of organic compounds, the basis of their molecules is made up of chains and rings formed from carbon atoms. Compounds containing only carbon and hydrogen are called hydrocarbons. In this case, part of the valences of carbon is spent on the formation of bonds with neighboring carbon atoms, and free valences bind carbon with hydrogen, oxygen, nitrogen, sulfur and, much less often, with other atoms of the periodic system. Very often, such a “skeleton” of carbon atoms is preserved as a result of chemical transformations undergone by a molecule of an organic compound, which greatly facilitates the prediction of the composition of products. Often reactions are limited to the replacement of one or more hydrogen atoms with another element or group of atoms (otherwise called a moiety or functional group ), resulting in an organic compound of a different class. Depending on the group that replaced one of the hydrogen atoms in the molecule of an organic compound as a result of the reaction, classes of organic compounds are distinguished.
Often, as a result of the reaction, one functional group is replaced by another, while maintaining the carbon skeleton. However, numerous reactions are also known that are accompanied by changes in the carbon skeleton of the molecule.
Table
Some functional groups of organic compounds
Functional group |
Group name |
Connection class |
General structure |
Examples |
- Cl , -F, -Br, -I (-X) |
Halogen |
Halides |
Bromobenzene |
|
Ethenyl chloride(vinyl chloride) |
||||
-HE |
Hydroxyl (hydroxy, hydroxy) |
Alcohols, phenols |
R-OH |
Phenol |
|
Methanol |
||||
> C=O |
Carbonyl (oxo) |
Aldehydes, ketones |
Propanone(acetone) Ethanal(acetaldehyde) |
|
-COUN |
Carboxyl (carboxy) |
Carboxylic acids |
Ethanovaacid (acetic acid) |
|
- NO 2 |
Nitro |
Nitro compounds |
Nitromethane |
|
-NH 2 |
Amino |
Amines |
Aminomethane(methylamine) |
|
-CN |
Cyano |
Nitriles |
Ethannitrile(acetonitrile) |
Homologues and homologous series
Homologs – organic compounds (of the same class, see above), differing in one or more methylene groups (-CH 2 - units). Homologues of alkanes are, for example, methane, ethane, propane, butane, etc., in which the number of carbon atoms changes by one (or by the same number of methylene units).
Homologues of aromatic compounds are benzene, toluene, xylenes, mesitylene, ethylbenzene and others alkyl-substituted benzenes. These compounds also differ in their gross formula by one or more methylene units (-CH 2 -). Accordingly, homologues are methanol, propanol and ethanol, acetone and methyl ethyl ketone, acetic and propionic acids, etc.
Isomerism of organic compounds
The structure formula (structural formula) describes the order of connection of atoms in a molecule, i.e. its chemical structure. Chemical bonds in the structural formula are represented by dashes. The bond between hydrogen and other atoms is usually not indicated (such formulas are called abbreviated structural formulas).
Structural formulas differ from molecular (gross) formulas, which show only which elements and in what proportion are included in the composition of the substance (i.e., qualitative and quantitative elemental composition), but do not reflect the order of bonding of atoms. For example, n-butane and isobutane have the same molecular formula C4H10, but a different sequence of connections.
Thus, the difference in substances is due not only to different qualitative and quantitative elemental compositions, but also to different chemical structures, which can only be reflected by structural formulas. Even before the creation of the theory of structure, substances with the same elemental composition, but with different properties, were known. Such substances were called isomers, and this phenomenon itself – isomerism. The basis of isomerism, as shown by A.M. Butlerov, lying difference in structure molecules consisting of the same set of atoms. Thus, isomerism is the phenomenon of the existence of compounds that have the same qualitative and quantitative composition, but different structures and, therefore, different properties.
For example, when a molecule contains 4 carbon atoms and 10 hydrogen atoms, two isomeric compounds can exist:

Depending on the nature of the differences in the structure of the isomers, there are structural And spatial isomerism.
Structural isomerism
Structural isomers – compounds of the same qualitative and quantitative composition, differing in the order of bonding of atoms, i.e. chemical structure.
For example, the composition C4H8 corresponds to 5 structural isomers:
Among organic compounds, the existence of a colossal number of only structural isomers is theoretically possible. Thus, among alkanes containing only carbon and hydrogen atoms, the number of possible isomers increases exponentially with increasing number of carbon atoms. If for a compound of composition C 4 H 10 only two isomers are possible, then for pentanes C 5 H 12 the number of such isomers increases to three, C 6 H 14 has 5 isomers, C 7 H 16 - 9 isomers, C 8 H 18 - 18 isomers, C 9 H 20 - 35 isomers, and for the pentacosane compound C 25 H 52, the existence of as many as 36,797,588 isomers is theoretically possible.
Using the example above, the following isomers can be distinguished:
- double bond positions (butene-1 and butene-2);
- carbon skeleton (butenes-1 and -2 and isobutylene);
- cycle sizes (cyclobutane and methylcyclopropane);
- interclass isomers (alkenes and cycloalkanes).
Interclass isomers are, for example, ethanol and dimethyl ether, which have the same gross formula C 2 H 6 O, but completely different structures and belong to different classes. They differ not only in their chemical properties (the more inert dimethyl ether does not react with sodium metal, unlike ethanol), but also in their physical properties. Ethanol is a liquid at normal temperature, while dimethyl ether is a gas.
Cyclic and acyclic organic compounds
It can be noted that among the structural isomers of organic compounds there may be molecules containing cycles built from carbon atoms different numbers (and often more than one such cycle in the molecule). On this basis they distinguish Ali cyclical connections (containing cycles, or simply cyclic connections) and A cyclical compounds (not containing cycles, but built exclusively from chains of carbon atoms, often branched).
Carbocyclic compounds contain only carbon atoms in the ring. They are divided into two groups with significantly different chemical properties: aliphatic cyclic (abbreviated alicyclic) And aromatic connections.
Heterocyclic compounds contain in the cycle, in addition to carbon atoms, one or more atoms of other elements – heteroatoms(from Greek heteros– other, other) – oxygen, nitrogen, sulfur, etc.
Spatial isomerism
Spatial isomers (geometric isomers, stereoisomers) with the same composition and the same chemical structure, they differ in the spatial arrangement of atoms in the molecule.
Spatial isomers are optical (mirror) and cis-trans- isomers. In the example shown above, 2-butene, which exists in nature as qi With - And trance- butenes-2:

Spatial isomerism occurs, in particular, when carbon has four different substituents:

If you swap any two of them, you get another spatial isomer of the same composition. The physicochemical properties of such isomers differ significantly. Compounds of this type are distinguished by their ability to rotate the plane of polarized light transmitted through a solution of such compounds by a certain amount. In this case, one isomer rotates the plane of polarized light in one direction, and its isomer rotates in the opposite direction. Due to such optical effects, this type of isomerism is called optical isomerism .
More details about optical isomerism can be found in the section on oxygen-containing and nitrogen-containing organic compounds.
Optical isomerism is a special case of spatial isomerism. Optical isomers are molecules that differ in the spatial arrangement of groups and atoms, having the same composition and the same order of atomic bonds. Solutions of such compounds are capable of rotating the plane of polarized light transmitted through them by a certain angle.
1.3.3. Nomenclature of organic compounds
Due to the presence of a huge number of organic compounds, the system of their designation (name) becomes of great importance so that by the name one can easily establish its structure (chemical structure), and, accordingly, all chemical and physical properties. Thus, the name should reflect the chemical structure of the organic compound as accurately as possible, including the ability to identify structural and geometric isomers. To date, three types of nomenclature of organic compounds have emerged:
1. trivial ;
2. rational ;
3. systematic (or substitution, or nomenclature IUPAC ).
The presence of trivial names is associated with history. Previously, researchers often named compounds based on the source of their isolation or on some organoleptic, physicochemical properties. Trivial names are sometimes in circulation with the same rights (if not more often) than systematic names. For example, the names acetic acid, formic acid, lactose, urea and many other names are still in use.
Rational nomenclature
This type of nomenclature has become widespread as a result of the fact that some compounds can be named as some kind of parent compound, from which they differ by substituents. An example would be neopentane (“new pentane”), a hydrocarbon of the alkanes class with the composition C5H12. The name “neopentane” is considered trivial, and says absolutely nothing about its structure. According to the second type of nomenclature, this hydrocarbon can be called tetramethylmethane. Name tetramethylmethane already much more informative in terms of information about the structure of the molecule. You can imagine a methane molecule in which all four hydrogen atoms are replaced by methyl groups.
Systematic the same name for neopentane is the name 2,2-dimethylpropane , compiled according to the rules developed by the International Union of Pure and Applied Chemistry (IUPAC - International Union of Pure and Applied Chemistry). The structural formula of neopentane is given below:
We will do a detailed consideration of the rules for naming organic compounds later, when considering individual classes of organic compounds, since each case has its own characteristics.
The replacement of hydrogen atoms in alkane molecules with any heteroatom (halogen, nitrogen, sulfur, oxygen, etc.) or group causes a redistribution of electron density. The nature of this phenomenon is different. It depends on the properties of the heteroatom (its electronegativity) and on the type of bonds along which this influence spreads.
Inductive effect
If the influence of a deputy is transferred through the participation s- bonds, then there is a gradual change in the electronic state of the bonds. This polarization is called inductive effect (I) , is represented by an arrow in the direction of the electron density shift. Electron density always shifts towards the MORE ELECTRONEGATIVE atom or group of atoms:
CH 3 -CH 2 -->Cl,
HO CH 2 -CH 2 --> Cl ,
CH 3 -CH 2 --> COOH,
CH 3 -CH 2 --> NO 2, etc.
The inductive effect is due to the desire of an atom or group of atoms to supply or withdraw electron density, and therefore it can be positive or negative. A negative inductive effect is exhibited by elements that are more electronegative than carbon, i.e. halogens, oxygen, nitrogen and others, as well as groups with a positive charge on the element associated with carbon. The negative inductive effect decreases from right to left in a period and from top to bottom in a group of the periodic system:
F > O > N,
F > Cl > Br > J.
In the case of fully charged substituents, the negative inductive effect increases with increasing electronegativity of the atom bonded to the carbon:
>O + - >> N +< .
In the case of complex substituents, the negative inductive effect is determined by the nature of the atoms that make up the substituent. In addition, the inductive effect depends on the nature of the hybridization of atoms. Thus, the electronegativity of carbon atoms depends on the hybridization of electron orbitals and changes in the following direction:
sp3< sp2 < sp .
Elements that are less electronegative than carbon exhibit a positive inductive effect; groups with a complete negative charge; alkyl groups. The +I-effect decreases in the series:
(SN 3 ) 3 C -> (CH 3) 2 CH-> CH 3 -CH 2 -> CH 3 -> H-.
The inductive effect of the substituent quickly decays as the chain length increases.
Table
Summary table of substituents and their electronic effects
X - halogen) |
Effects |
CH 3 > CH 3 -CH 2 - > (CH 3) 2 CH- >> CH 2 X |
I, +M |
(CH 3) 3 C- |
I, M = 0 |
Atom attached top- X- (halogen ), -O - , -OH, -OR, -NH 2 , -NHR, -NR 2 , -SH, -SR, |
–I, +M |
attached top- CHX 2, -CX 3, -C=N=S |
–I, –M |
More electronegative carbon (compared to sp3): CH=C N- , -S = (but easily transmits the M-effect in any direction) |
–I, M = 0 |
|
N + H 3, -N + R 3, (-S + R 2, -O + H 2), |
–I, M = 0 |
Mesomeric effect
The presence of a substituent with a free pair of electrons or a vacant p-orbital attached to a system containing p-electrons leads to the possibility of mixing the p-orbitals of the substituent (occupied or vacant) with p-orbitals and a redistribution of electron density in compounds. This effect is called mesomeric .
The shift in electron density is usually insignificant and bond lengths remain virtually unchanged. A slight shift in the electron density is judged by the dipole moments, which are small even in the case of large conjugation effects on the outer atoms of the conjugated system.
The mesomeric effect is depicted by a curved arrow directed towards the shift in electron density. Electron density always shifts to the side more electronegative atom located at the edge of the structure and connected to the rest of the structure multiple connection:

Depending on the direction of displacement of the electron cloud, the mesomeric effect can be positive (+M), an atom, or when a group of atoms transfer electrons to the pi system:
and negative (- M) when a group of atoms pulls electrons from the pi system:

The positive mesomeric effect (+M) decreases with an increase in the electronegativity of the atom carrying a lone pair of electrons, due to a decrease in the tendency to donate it, as well as with an increase in the volume of the atom. The positive mesomeric effect of halogens changes in the following direction:
F> Cl > Br> J (+M -effect).
Groups with lone pairs of electrons on the atom attached to the conjugate have a positive mesomeric effect. pi-system:
- NH2( NHR, NR 2) > OH( OR ) > X (halogen) (+M-effect).
The positive mesomeric effect decreases if the atom is bonded to an electron acceptor group:
-NH 2 > -NH-CO-CH 3 .
The negative mesomeric effect increases with increasing electronegativity of the atom and reaches maximum values if the acceptor atom carries a charge:
>C=O + H >> >C=O.
A decrease in the negative mesomeric effect is observed if the acceptor group is conjugated with a donor group:
-CO-O - << - СО -NH 2< -CO-OR < -CO-H(R) << -CO- CO- < -CO-X ( halogen ) (– M-effect).
Table
Substituent or group of atoms ( X - halogen) |
Effects |
CH 3 > CH 3 -CH 2 - > (CH 3) 2 CH- >> CH 2 X |
I, +M |
(CH 3) 3 C- |
I, M = 0 |
Atom attached top-system, has a lone pair of electrons: X- (halogen |
–I, +M |
attached top-system, the atom, in turn, is connected to a more electronegative atom: N=O, -NO 2 , -SO 3 H, -COOH, -CO-H, -CO-R, -CO-OR, -CN, - CHX 2, -CX 3, -C=N=S |
–I, –M |
|
CH=C N- , -S = CH (ethynyl), -C 6 H 4 - (phenylene) |
–I, M = 0 |
An atom having no p orbitals, but with a total positive charge |
–I, M = 0 |
Hyperconjugation or superconjugation
An effect similar to positive mesomeric occurs when the hydrogen at a multiple bond is replaced by an alkyl group. This effect is directed towards the multiple bond and is called hyperconjugation(superconjugate):
The effect resembles a positive mesomeric one, since it donates electrons to the conjugate p- system:

Superconjugation decreases in the sequence:
CH 3 > CH 3 -CH 2 > (CH 3) 2 CH > (CH 3) 3 C.
To show the effect hyperconjugation it is necessary to have at least one hydrogen atom at the carbon atom adjacent to the p-system. The tert-butyl group does not exhibit this effect, and therefore its mesomeric effect is zero.
Table
Summary table of substituents and their electronic effects
Substituent or group of atoms ( X - halogen) |
Effects |
CH 3 > CH 3 -CH 2 - > (CH 3) 2 CH- >> CH 2 X |
I, +M |
(CH 3) 3 C- |
I, M = 0 |
Atom attached top-system, has a lone pair of electrons: X- (halogen ), -O - , -OH, -OR, -NH 2 , -NHR, -NR 2 , -SH, -SR, |
–I, +M |
attached top-system, the atom, in turn, is connected to a more electronegative atom: N=O, -NO 2 , -SO 3 H, -COOH, -CO-H, -CO-R, -CO-OR, -CN, - CHX 2, -CX 3, -C=N=S |
–I, –M |
More electronegative carbon: CH=C N- , -S = CH (ethynyl), -C 6 H 4 - (phenylene) (but easily transmits the M-effect in any direction) |
–I, M = 0 |
An atom that has no p orbitals but has a total positive charge N + H 3, -N + R 3, (-S + R 2, -O + H 2), |
Most organic compounds contain only a few basic elements: carbon, hydrogen, nitrogen, oxygen, sulfur and, much less frequently, other elements. Thus, the entire diversity of organic compounds is determined, on the one hand, by their qualitative and quantitative composition, and on the other, by the order and nature of the bonds between atoms. 1.1 Electronegativity of elements The electronegativity of an atom is its ability to attract elements. Electronegativity values are not significant constants, but only show the relative ability of atoms to attract electrons more or less when forming with other atoms. Atoms located in the electronegativity series in front of carbon and having an electronegativity value less than 2.5 increase the electron density on the carbon atom when forming a bond with it. On the contrary, atoms whose electronegativity value exceeds 2.5 reduce the electron density on the carbon atom when forming a bond. 1.2 Ionic bonding The electronic configuration for any atom can be formed in two different ways. One of them is electron transfer: atoms of one element give up electrons, which go to the atoms of another element. In this case, a so-called ionic (electrovalent, heteropolar) bond: An atom that donates electrons becomes a positive ion ( cation); an atom that has accepted an electron becomes a negative ion ( anion). Distinctive features of ionic compounds are the instantaneous reaction, dissociation and solvation of ions in aqueous solutions, high melting and boiling points, solubility in polar solvents, electrical conductivity of solutions and melts. A heteropolar bond occurs between atoms that differ greatly in electronegativity. 1.3 Covalent bond When atoms of equal or similar electronegativity interact, electron transfer does not occur. The formation of an electronic configuration for such atoms occurs due to the generalization of two, four or six electrons by interacting atoms. Each of the generalized pairs of electrons forms one covalent (homeopolar) bond: The most important physical parameters of a covalent bond are those that characterize their symmetry, size, electrical and thermochemical properties. Link length- this is the equilibrium distance between the centers of nuclei and it depends on what other atoms they are associated with. Thus, the length of the C-C bond, depending on the environment, varies within the range of 0.154 – 0.14 nm. Bond angles– angles between lines connecting bonded atoms. Knowledge of bond lengths and bond angles is necessary for constructing a correct spatial model, an idea of the electron density distribution, and is used in quantum chemical calculations. Energy of breaking a chemical bond is the energy spent on breaking this bond or released during its formation per mole of particles. In the case of molecules containing two or more identical bonds, the energy of breaking one of these bonds or the average energy of breaking these bonds is distinguished. The higher the chemical bond energy, the stronger the bond. A bond is considered strong, or strong, if its energy exceeds 500 kJ/mol, weak - if its energy is less than 100 kJ/mol. If the interaction of atoms releases energy less than 15 kJ/mol, then it is considered that a chemical bond is not formed, but intermolecular interaction is observed. Bond strength generally decreases as bond length increases. Polarity of chemical bonds– characteristic of a chemical bond, showing a change in the distribution of electron density in the space around the nuclei in comparison with the distribution of electron density in the neutral atoms forming this bond. Knowledge of bond polarity is necessary to judge the distribution of electron density in a molecule, and therefore the nature of its reactivity. Bond polarizability is expressed in the displacement of bond electrons under the influence of an external electric field, including that of another reacting particle. Polarizability is determined by electron mobility. Electrons are more mobile the further they are from the nuclei. 1.4 Breaking ties Breaking a covalent bond between two atoms can occur in different ways: When A each atom is separated with one electron, resulting in the formation of particles called radicals, which are highly reactive due to the presence of an unpaired electron; such a gap is called homolytic cleavage communications. In cases b And V one atom can hold both electrons, leaving the other atom without electrons, resulting in negative and positive ions, respectively. If the R and X atoms are not identical, splitting can occur along one of these paths, depending on which atom - R or X - holds a pair of electrons. These kinds of gaps are called heterolytic cleavage and lead to the formation of an ion pair. Share with friends or save for yourself:
|