How and where is the process of photosynthesis in plants? Phases photosynthesis Light dark reaction.
Using light energy or without it. It is characteristic of plants. Let us then consider what is the dark and light phase of photosynthesis.
General
The photosynthesis organ in higher plants is a sheet. Chloroplasts are organoids as organoids. In the membranes of their thylacoids there are photosynthetic pigments. They are carotenoids and chlorophylls. The latter exist in several species (A, C, B, D). The main one is considered a-chlorophyll. In its molecule, the porphyrine "head" is released with a magnesium atom located in the center, as well as the phytolon "tail". The first element is represented as a flat structure. The head is hydrophilic, therefore it is located on the part of the membrane that is directed to the aquatic environment. The phytolon "tail" is hydrophobic. Due to this, it holds a chlorophille molecule in the membrane. Chlorophylls are absorbed by blue-purple and red light. They also reflect green, due to which plants are characteristic of them color. In the pylactotoid membranes, chlorophyll molecule is organized in photosystems. For syneselen algae and plants are characterized by systems 1 and 2. Photosyntheses of bacteria have only the first. The second system can decompose H 2 O, separating the oxygen.

Light phase photosynthesis
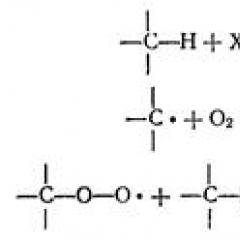
The processes occurring in plants are distinguished by complexity and multistage. In particular, two groups of reactions are distinguished. They are the inamic and light phase of photosynthesis. The latter occurs with the participation of the enzyme ATP, proteins carrying electrons, and chlorophyll. The light phase of photosynthesis occurs in tilactoid membranes. Chlorophile electrons are excited and leave the molecule. After that, they fall on the outer surface of the pylactotoid membrane. She, in turn, is charging negatively. After oxidation, the restoration of chlorophyll molecules begins. They select electrons in water, which is present in intracathic space. Thus, the light phase of photosynthesis proceeds in the membrane during decay (photolidium): H 2 O + Q light → H + + it is
The hydroxyl ions are converted to reactive radicals, giving their electrons:
He - → .on + e -
He-radicals are combined and form free oxygen and water:
4 But → 2N 2 O + O 2.

At the same time, oxygen is removed into the surrounding (outer) medium, and the protons accumulation in a special "tank" is accumulated inside the tilactoid. As a result, where the light phase of photosynthesis flows, the pylactotoid membrane due to N + on one side receives a positive charge. At the same time, at the expense of electrons, it charges negatively.
Phosphylation ADF
Where the light phase of photosynthesis flows, there is a potential difference between the inner and outer surfaces of the membrane. When it reaches 200 mV, protone pushing through the ATP synthetase channels. Thus, the light phase of photosynthesis occurs in the membrane during phosphorylation ADF to ATP. At the same time, atomic hydrogen is directed to the restoration of a special carrier nicotNADF + to NAPF.N2:
2N + + 2e - + Nadf → NADF.N 2
The light phase of photosynthesis, thus, includes a photoliz of water. It, in turn, accompany the three most important reactions:
- Synthesis ATP.
- EDUCATION NADF.N 2.
- Formation of oxygen.
The light phase of photosynthesis is accompanied by the release of the latter into the atmosphere. NADF.N2 and ATP move in the stromom of chloroplast. This light phase of photosynthesis is completed.

Another group of reactions
For the dark phase of photosynthesis, light energy is not needed. It goes in the stroma of chloroplast. The reactions are represented as a chain of consistently occurring transformations from air carbon dioxide. As a result, glucose and other organic substances are formed. The first reaction is fixation. As an acceptor carbon dioxide, ribulosobiphosphate (five-carbon sugar) ribf performs. The catalyst in the reaction is ribulosobyphosphate carboxylase (enzyme). As a result of carboxylation, the Ribf is formed by a six-curved unstable connection. It practically instantly disintegrates into two FGK (phosphoglycerolic acid) molecules. After that, there is a cycle of reactions, where it is transformed into glucose through several intermediate products. They use ENERGY NADF.N 2 and ATP, which were transformed when the light phase of photosynthesis was covered. The cycle of these reactions is referred to as the Calvin cycle. It can be represented as follows:
6SO 2 + 24H + + ATP → C 6H 12 ° 6 + 6N 2
In addition to glucose, during photosynthesis, other organic (complex) compounds are formed. These, in particular, are fatty acids, glycerin, nucleotide amino acids.

C3 reactions
They represent the type of photosynthesis, in which three-carbon compounds are formed as the first product. It is it described above as a Calvin cycle. As characteristic features of C3-photosynthesis are:
- The Ribf is an acceptor for carbon dioxide.
- The carboxylation reaction catalyzes the ribf carboxylase.
- A hexagonnel substance is formed, which subsequently disintegrates on 2 FGK.
Phosphoglycerin acid is restored to TF (trioseophosphates). Some of them are directed to the regeneration of ribulosobiphosphate, and the rest - turns into glucose.
C4 reactions
For this type of photosynthesis, the appearance of quadruple compounds as the first product is characteristic. In 1965, it was revealed that C4 substances appear first in some plants. For example, it was found for millet, sorghum, sugar cane, corn. These cultures began to refer to C4 plants. The following, 1966, Slek and Hatch (Australian scientists) revealed that they almost completely absent photography. It was also found that such C4 plants are much more efficiently carried out by absorption of carbon dioxide. As a result, the path of carbon transformation in such cultures began to be called the Hatch Salca.

Conclusion
The value of photosynthesis is very large. Thanks to him, carbon dioxide gas is absorbed annually from the atmosphere (billions of tons). Instead, it is distinguished by no less oxygen. Photosynthesis acts as the main source of formation of organic compounds. Oxygen is involved in the formation of the ozone layer, providing the protection of living organisms from the effects of short-wave UV radiation. In the process of photosynthesis, the sheet absorbs only 1% of the entire energy of the light falling on it. Its productivity is within 1 g of organic compound per 1 kV. m surface per hour.
Photosynthesis consists of two phases - light and dark.
In the light phase, the light quanta (photons) interact with chlorophyll molecules, as a result of which these molecules are moving at a very short time in a richer energy, "excited" state. Then the excess energy of the part of the "excited" molecules is converted into heat or emitted as light. Another part of it is transmitted by hydrogen ions, always available in an aqueous solution due to water dissociation. The formed hydrogen atoms are fragile with organic molecules - hydrogen carriers. Hydroxide ions "give their electrons to other molecules and turn into free radicals. He interacts with each other, resulting in water and molecular oxygen:
4one \u003d O2 + 2N2O Thus, a source of molecular oxygen, which is generated during photosynthesis and released into the atmosphere, is a photoliz - water decomposition under the influence of light. In addition to photolysis of water, the energy of solar radiation is used in the light phase for the synthesis of ATP and ADP and phosphate without oxygen participation. This is a very effective process: in chloroplasts is formed 30 times more ATPs than in the mitochondria of the same plants involving oxygen. In this way, the energy required for the processes in the dark phase of photosynthesis is accumulated.
In the complex of chemical reactions of the dark phase, for the flow of which the light is not required, the key place is bonding CO2. In these reactions, ATP molecules are involved, synthesized during the light phase, and hydrogen atoms formed during the photolysis of water and associated with carrier molecules:
6So2 + 24N - »C6H12O6 + 6NEO
So the energy of sunlight is converted into the energy of chemical bonds of complex organic compounds.
87. The value of photosynthesis for plants and for the planet.
Photosynthesis is the main source of biological energy, photosynthetic autotrophs use it for the synthesis of organic substances from inorganic, heterotrophs exist due to energy stored by autotrophs in the form of chemical bonds, released it in breathing and fermentation processes. The energy obtained by humanity during the combustion of fossil fuels (coal, oil, natural gas, peat) is also stored in the process of photosynthesis.
Photosynthesis is the main entrance of inorganic carbon into the biological cycle. All free oxygen atmosphere - biogenic origin and is a by-product of photosynthesis. The formation of an oxidative atmosphere (oxygen catastrophe) completely changed the state of the earth's surface, made it possible to appear breathing, and in the future, after the formation of the ozone layer, made it possible to reach the land. The process of photosynthesis is the basis of nutrition of all living beings, and also supplies humanity with fuel (wood, coal, oil), fibers (cellulose) and countless useful chemical compounds. Of the carbon dioxide and water connected from the air during photosynthesis, about 90-95% of the dry weight of the harvest is formed. The remaining 5-10% fall on mineral salts and nitrogen derived from the soil.
A person uses about 7% of photosynthesis products in food, as animal feed and in the form of fuel and building materials.
Photosynthesis, which is one of the most common processes on Earth, determines the natural cycle of carbon, oxygen and other elements and ensures the material and energy basis of life on our planet. Photosynthesis is the only source of atmospheric oxygen.
Photosynthesis is one of the most common processes on Earth, causes a circulation in the nature of carbon, O2 and other elements. It makes up the material and energy basis of everything alive on the planet. Every year, as a result of photosynthesis, about 8 1010 tons of carbon binds in the form of an organic substance, up to 1011 tons of cellulose is formed. Thanks to the photosynthesis of sushi plants form about 1.8 1011 tons of dry biomass per year; Approximately the same amount of biomass of plants is formed annually in the World Ocean. The rainforest brings up to 29% into the overall products of the photosynthesis of land, and the contribution of forests of all types is 68%. The photosynthesis of higher plants and algae is the only source of atmospheric O2. The emergence on Earth is about 2.8 billion years ago, the mechanism of water oxidation with the formation of O2 is the most important event in biological evolution, which made the light of the Sun by the main source - the free energy of the biosphere, and water is a practically unlimited source of hydrogen for the synthesis of substances in living organisms. As a result, an atmosphere of modern composition was formed, O2 became accessible to oxidation of food, and this led to the occurrence of highly organized heterotrophic organisms (used as an exogenous organic substances as a carbon source). The overall stock of solar radiation energy in the form of photosynthesis products is about 1.6,1021 kJ per year, which is about 10 times higher than the modern energy consumption of humanity. Approximately half of the solar radiation energy accounts for a visible range of spectrum (wavelength l from 400 to 700 nm), which is used for photosynthesis (physiologically active radiation, or headlights). IR radiation is not suitable for photosynthesis of oxygening organisms (higher plants and algae), but is used by some photosynthetic bacteria.
Opening of the process of Chemosynthesis S.N. Vinogradsky. Process characteristic.
Chemosynthesis is a process of synthesizing from carbon dioxide organic substances, which occurs due to the energy separated during the oxidation of ammonia, hydrogen sulfide and other chemicals, during the life of microorganisms. Chemosynthesis also has another name - chemolyteavotrophy. The opening of Chemosynthesis S. N. Vinogradovsky in 1887, the root changed the representations of the science of the type of metabolism, which are the main organisms. Chemosynthesis for many microorganisms is the only type of food, as they are able to absorb carbon dioxide as the only carbon source. Unlike photosynthesis in chemosynthesis, an energy that is formed as a result of oxidative reaction reactions is used instead of light energy.
This energy should be sufficient for the synthesis of adenosineryphosphoric acid (ATP), and its number must exceed 10 kcal / mol. Some of the oxidized substances give their electrons into the chain at the cytochrome level, and thus created for the redundant synthesis additional energy consumption. With chemosynthesis, the biosynthesis of organic compounds occurs due to the authotrophic assimilation of carbon dioxide, that is, in the same way as at photosynthesis. As a result of the transfer of electrons by the chain of the respiratory enzymes of bacteria, which are built into the cell membrane, the energy in the form of ATP is obtained. Due to the very large energy consumption, all chemosynthetic bacteria, except hydrogen, form quite little biomass, but at the same time they oxidize the large amount of inorganic substances. Hydrogen bacteria are used by scientists to obtain protein and purification of the atmosphere from carbon dioxide, this is especially necessary in closed environmental systems. There is a great diversity of chemosynthetic bacteria, most of them relate to pseudomonads, they are also found among the filamentons and binding bacteria, leptospir, Spirill and Corinbacteria.
Examples of use of chemosynthesis prokaryotm.
The essence of chemosynthesis (the process opened by the Russian researcher Sergey Nikolayevich Vinogradsky) is the production of energy by the organism by the organism of the oxidation reactions carried out by this organism themselves with simple (inorganic) substances. Examples of such reactions may be an ammonium oxidation to nitrite, or bivalent iron to a triturable, hydrogen sulfide to sulfur, and the like-specific prokaryotic groups (bacteria in the broad sense of the word) are capable of chemosynthesis (bacteria in a broad sense of the word). Due to Chemosynthesis, there are currently only ecosystems of some hydrothermals (places at the bottom of the ocean, where there are yields of hot groundwater, rich in restored substances - hydrogen, hydrogen sulfide, iron sulphide, etc.), as well as extremely simple, consisting only of bacteria , Ecosystems discovered at great depths in the rims of rocks on land.
Bacteria - chemosynthetics, destroy rocks, purify wastewater, participate in the formation of minerals.
Topic 3 Stages of photosynthesis
Section 3 photosynthesis
1. Light phase photosynthesis
2.Photosynthetic phosphorylation
3. Putting CO 2 at photosynthesis
4. Tripping
The essence of the light phase of photosynthesis is to absorb the radiant energy and its transformation into the assimilative force (ATP and NADF-H), which is necessary to restore carbon in the dark reactions. The complexity of the transformation of light energy transform to the chemical requires their strict membrane organization. The light phase of photosynthesis occurs in chloroplast marins.
Thus, the photosynthetic membrane carries out a very important reaction: it turns the energy of the absorbed light quanta into the oxidation-reduction potential of NADF-H and into the potential of the phosphoryl group transfer reaction in the ATP molecule, at the same time, the energy conversion is converted from very short-lived form in the form sufficiently long-lived. Stabilized energy can be later used in biochemical reactions of plant cell, including in reactions leading to the restoration of carbon dioxide.
Five main polypeptide complexes are built into the inner membranes of chloroplasts: fotosystem I (FS I) Complex, Photosystem II (FSII) Complex, Light Coaching Complex II (SSKII), Cytochromic B 6 F-complex and ATP synthase (CF 0 - CF 1 -complex). FSI complexes, FSII and SSKII contain pigments (chlorophylls, carotenoids), most of which are functioning as antenna pigments, collecting energy for the pigments of FSI and FSII reaction centers. FSI and FSII complexes, as well as cytochromic b 6 F. -complex are in its composition redox-cofactors and are involved in the photosynthetic transport of electrons. The proteins of these complexes are distinguished by a high content of hydrophobic amino acids, which ensures their embedding in the membrane. ATP synthase ( CF 0 - CF 1 -complex) carries out the synthesis of ATP. In addition to large polypeptide complexes in Tylacoid membranes there are small protein components - plastociana, ferredoxin and ferredoxin-nadf-oxidoreductase, Located on the surface of the membranes. They enter the electronic transport system of photosynthesis.
The following processes occur in the light cycle of photosynthesis: 1) photoexcitation of photosynthetic pigment molecules; 2) energy migration from antenna to the reaction center; 3) photocosis of the water molecule and the selection of oxygen; 4) photo sealing NADF to Nadf-N; 5) Photosynthetic phosphorylation, formation of ATP.
Pigments of chloroplasts are combined into functional complexes - Pigment systems in which the reaction center - chlorophyll but, Carrying out photosensitization is connected by energy transfer processes with an antenna consisting of light-cutting pigments. The modern scheme of photosynthesis of higher plants includes two photochemical reactions carried out with the participation of two different photosystems. The assumption of their existence was expressed by R. Emerson in 1957, on the basis of the effect of long-wavelength red light detected by him (700 nm), joint illumination of more short-wave rays (650 nm). Subsequently, it was found that the photosystem II absorbs more short-wave rays compared to the FSI. Photosynthesis is effective only when they are jointly functioning, which explains Emerson's strengthening effect.
The composition of the FSI, chlorophyll dimer is included as a reactionary center a C. Maximum absorption of light 700 nm (p 700), as well as chlorophylls but 675-695, playing the role of an antenna component. The primary electron acceptor in this system is the monomeric form of chlorophyll but 695, secondary acceptors - iron-top proteins (-fes). The FSI complex under the action of light restores the iron-containing protein - ferredoxin (PD) and oxidizes the copper-containing protein - plastocianine (PC).
FSII includes a reaction center containing chlorophyll but(P 680) and antenna pigments - chlorophylls but 670-683. The primary electron acceptor is faeofitin (FF) transmitting electrons to the plastochinone. The FSII also includes the protein complex of the S-system, oxidizing water, and the carrier of electrons Z. This complex is functioning with the participation of manganese, chlorine and magnesium. FSII restores plastochinone (PQ) and oxidizes water with highlighting about 2 and protons.
The link between FSII and FSI serve as the Fund of Plastokhinonov, protein cytochromic complex b 6 F. and plastocianine.
In chloroplasts of plants, each reactionary center accounts for about 300 molecules of pigments, which are included in the antenna or light-cutting complexes. From the chloroplast lamella, a light-cutting protein complex containing chlorophylls is isolated but and b. and carotenoids (SSC), closely associated with FSP, and antenna complexes directly included in the FSI and FSII (focusing the antenna components of the photosystems). Half of the protein of thylakoids and about 60% chlorophyll is localized in the SSK. Each SSC contains from 120 to 240 chlorophyll molecules.
The antenna protein complex FS1 contains 110 chlorophyll molecules a. 680-695 per p 700 , Of these, 60 molecules are the components of the antenna complex, which can be viewed as SSC FSI. The antenna complex FSI also contains B-carotene.
The antenna protein complex FSII contains 40 chlorophyll molecules butwith a maximum of absorption of 670-683 nm per r 680 and B-carotene.
Chromoproteins of the antenna complexes do not have photochemical activity. Their role is to absorb and transmit the energy of quanta on a small amount of molecules of reaction centers R 700 and P 680, each of which is associated with the electron transport chain and performs a photochemical reaction. The organization of electron-vehicle chains (ETC) with all chlorophyll molecules is irrational, since even on the direct sunlight on the pigment molecule, the luminous quanta falls more than once in 0.1 s.
Physical mechanisms of the processes of absorption, stocks and migration of energy Chlorophyll molecules are well studied. Absorption of photon (hν) is due to the transition of the system to various energy states. In the molecule, in contrast to the atom, electronic, oscillatory and rotational movements are possible, and the total energy of the molecule is equal to the sum of these types of energies. The main indicator of the energy absorbing system is the level of its electronic energy, is determined by the energy of external electrons in orbit. According to Pauli's principle, there are two electrons with oppositely directed backs, as a result of which a stable system of paired electrons is formed. The absorption of light energy is accompanied by the transition of one of the electrons to a higher orbit with the stock of the absorbed energy as an electronic excitation energy. The most important characteristic of the absorbing systems is the selectivity of the absorption, determined by the electronic configuration of the molecule. In a complex organic molecule, there is a certain set of free orbits, for which the electron transition is possible when absorbed by the light quanta. According to the "frequencies" of the boron, the frequency of the absorbed or emitted radiation V should strictly correspond to the difference of energies between the levels:
ν \u003d (E 2 - E 1) / H,
where h is a constant plank.
Each electronic transition corresponds to a certain absorption band. Thus, the electronic structure of the molecule determines the nature of electron-oscillatory spectra.
Passenger of absorbed energy associated with the emergence of electronically excited states of pigments. The physical patterns of excited states of Mg-porphyrins can be considered based on the analysis of the electronic transition scheme of these pigments (drawing).
Two main types of excited states are known - singlet and triplet. They differ in energy and the state of the electron spin. In the singlet excited state, the spins of electrons at the main and excited levels remain anti-parallel, during the transition to the triplet state, the spin of the excited electron is rotated to form a biradical system. When the photon is absorbed by the chlorophyll molecule, it moves from the main (S 0) to one of the excited singlet states - S 1 or S 2 , What is accompanied by an electron transition to an excited level with higher energy. The excited state S 2 is very unstable. The electron quickly (for 10 -12 c) loses a part of the energy in the form of heat and falls to the lower oscillatory level S 1, where it may be within 10 -9 s. In a state S 1, an electron spin appeal may occur and transition to triplet state T 1, the energy of which is lower than S 1 .
Several ways of deactivation of excited states are possible:
· Photon radiation with system transition to the ground state (fluorescence or phosphorescence);
· Energy transfer to another molecule;
· Use of excitation energy in a photochemical reaction.
Migration of energy Between pigment molecules can be carried out in the following mechanisms. Inductive resonance mechanism (Furster mechanism) is possible provided when the electron transition is optically allowed and the exchange of energy is carried out by exciton mechanism. The concept of "EXITON" means the electronically excited state of the molecule, where the excited electron remains associated with the molecule of the pigment and the separation of charges does not occur. Energy transfer from the excited pigment molecule to another molecule is carried out by nonradiative transfer of excitation energy. An electron electron is an oscillating dipole. The variable electrical field forming can cause similar electron oscillations in another pigment molecule when performing resonance conditions (energy equality between the main and excited levels) and the induction conditions that determine quite strong interaction between molecules (no more than 10 nm).
Exchange-resonant Mechanism of Migration of Tereny Dexter Energy It takes place when the transition is optically forbidden and the dipole during the excitation of the pigment is not formed. It requires a close contact of molecules (about 1 nm) with overlapping external orbital. Under these conditions, the exchange of electrons located both on singlet and triplet levels is possible.
There is a concept about photochemistry quantum flow process. With reference to photosynthesis, this indicator of the effectiveness of the transformation of light energy into chemical energy shows how many quanta light is absorbed in order for one molecule about 2. It should be borne in mind that each photo active substance molecule simultaneously absorbs only one square of light. This energy is enough to cause certain changes in the photo active substance molecule.
The value, reverse quantum consumption, is called quantum outlet: The number of selected oxygen molecules or absorbed carbon dioxide molecules occurring on one quantum of light. This indicator is less than one. So, if 8 molecules of CO 2 consumes 8 light quanta, then the quantum yield is 0.125.
The structure of the electron-transport chain of photosynthesis and the characteristic of its components.The electron-transport chain of photosynthesis includes a rather large number of components located in the membrane structures of chloroplasts. Almost all components, in addition to quinions, are proteins containing functional groups capable of reversible reduction changes, and performing the functions of electrons or electrons in conjunction with protons. A number of ETC carriers include metals (iron, copper, manganese). As the most important components of electron transfer in photosynthesis, the following groups of compounds can be noted: cytochromes, quinones, pyridinnucleotides, flavoproteins, as well as ironoproteins, copperproteins and manganesprotein. The location of the named groups in ETC is determined primarily by their oxidative and recovery potential.
Presentations on photosynthesis, during which oxygen is released, was formed under the influence of the Z-scheme of electronic transport R. Hill and F. Bendella. This scheme was presented on the basis of measuring the oxidative and reducing potentials of cytochromes in chloroplasts. The electron-vehicle circuit is the transformation of the electron physical energy into the chemical bond energy and includes FS I and FS II. The Z-scheme comes from the sequential functioning and combining FSII with FSI.
P 700 is a primary electron donor, is chlorophyll (according to some data - dimer chlorophyll a), transmits an electron to an intermediate acceptor and can be oxidized with a photochemical path. A 0 - an intermediate electron acceptor - is a chlorophyll dimer.
Secondary electron acceptors are bound ironing centers A and V. An element of the structure of iron-arm proteins is a lattice from interrelated iron and sulfur atoms, which is called a ironer cluster.
Ferredoxin, soluble in the stromal phase of the chloroplast of the iron-protein, which is outside the membrane, transfers the electrons from the FSI reactionary center to the NADF as a result, the NADF-H is formed necessary for fixing CO 2. All soluble ferredoxins of photosynthetic organisms separating oxygen (including cyanobacteria) refer to the type 2fe-2S.
The electrons carrying component is also cytochrome F associated with the membrane. The electron acceptor for the cytochrome-fed membrane and the direct donor for the chlorophyll-protein complex of the reaction center is the copper-containing protein, which is called the "distribution carrier" - plastocian.
Chloroplasts also contain cytochrome B 6, and b 559. Cytochrome B 6, which is a polypeptide with a molecular weight of 18 kDa, is involved in the cyclic transfer of an electron.
Complex B 6 / F is an integral membrane complex of polypeptides containing cytochrome type B and F. The cytochrome complex B 6 / F catalyzes the transport of electrons between the two photosystems.
The cytochrome complex B 6 / F restores a small pool of water-soluble metalloprotein - plastocianine (PC), which serves to transmit reduction equivalents to the FS I. complex. Plastocianin is a small hydrophobic metalloprotein, which includes copper atoms.
Participants in the primary reactions in the reaction center of the FS II is the primary donor of the electrons P 680, the intermediate acceptor of theofin and two plastochinone (usually denoted q and c), located close to Fe 2+. The primary donor of the electrons is one of the forms of chlorophyll A, called P 680, since a significant change in light absorption was observed at 680.
The primary electron acceptor in the FS II is plastochinone. It is assumed that q is an iron-chinon complex. The secondary electron acceptor in the FS II is also a plasticon denoted by B, and functioning sequentially with Q. Plasticone / Plastokhinon system tolerates simultaneously with two electrons two more protons and in connection with this is a two-electron redox system. As the two electrons are transmitted by ETC through the plastochinone / plasticone system, two protons are transferred through a tylacoid membrane. It is believed that the proton concentration gradient arising from this, and is the driving force of the ATP synthesis process. The consequence of this is to increase the concentration of protons inside the thylacoids and the occurrence of a significant gradient of the pH between the outer and the inner side of the thylacoid membrane: from the inner side of the medium is more acidic than from the external.
2. Photosynthetic phosphorylation
Electron donor for FS-2 is water. Water molecules, giving electrons, disintegrate on the free hydroxyl, and proton H +. Free hydroxyl radicals, reacting with each other, give H 2 O and O 2. It is assumed that manganese and chlorine ions are involved in photocructuring water as cofactors.
In the process of photolisis of water, the essence of photochemical work carried out during photosynthesis is manifested. But the oxidation of water occurs under the condition that the electron electron is transmitted from the molecule. In the ETC photosystems-2, plastochinone, cytochromas, plastocianine (protein containing copper), FAD, NADF, etc. are served by electron carriers.
Embossed from the molecule P 700 electron is captured by a protein containing iron and sulfur, and is transmitted to ferredoxin. In the future, the path of this electron can be dual. One of these paths consists of an alternate transfer of an electron from ferredoxin through a row of carriers again to P 700. Then the quantum of light knocks down the following electron from the P 700 molecule. This electron comes to ferredoxin and returns to the chlorophyll molecule again. The cyclicity of the process is clearly traced. When the electron is transferred from ferredoxin, the electronic excitation energy goes to the formation of ATF from ADP and N Z P0 4. This type of photo phosphorylation named R. Arnon cyclical . Cyclic photo phosphaeling can theoretically flow and with closed dusts, because it is optional to exchange with the atmosphere.
Non-cyclic photo phosphorylation proceeds with the participation of both photosystems. In this case, the electrons are knocked out of P 700 and the proton H + comes to ferredoxin and is transferred through a row of carriers (phad, etc.) on the NADF with the formation of the reduced NAPF · H 2. The latter, like a strong reducing agent, is used in dark photosynthesis reactions. At the same time, the chlorophyll molecule P 680, absorbing the quantum of light, also goes into an excited state, giving one electron. Having passed through a series of carriers, the electron replenishes electron failure in the molecule No. 700. The electronic "hole" of chlorophyll P 680 is replenished due to the electron from the ion, one of the water photo polyesis products. The electron energy, knocked out by a quantum of light from P 680, during the transition through the electro-transport chain to the photo system 1 goes to the implementation of photophosphorylation. With non-cyclically electron transport, as can be seen from the circuit, the water photo and the release of free oxygen occurs.
Electron transfer is the basis of the photo phosphorylation mechanism considered. The English biochemist P. Mitchell put forward the theory of photophosphorylation, the name of the chemiosmotic theory. Etz chloroplasts, as you know, is located in the membrane of Tylacoid. One of the carriers of electrons into the ETC (Plastokhinon), according to the hypothesis of P. Mitchell, transfers not only electrons, but also protons (H +), moving them through the tylacoid membrane in the direction outside inside. Inside the tilacoid membrane with the accumulation of protons, the medium is acidified and in connection with this, the pH gradient arises: the outer side becomes less acidic than the inner. This gradient also increases due to the arrival of protons - water photo tag products.
The pH difference between the outer side of the membrane and the inner creates a significant source of energy. With this energy, the protons for special channels in special mushroom growth on the outside of the tylacide membrane are emitted out. In these channels there is a pairing factor (special protein), which is able to take part in photo phosphaeling. It is assumed that such a protein is a fusion enzyme that catalyzing the response of ATP decay, but in the presence of energy flowing through the proton membrane - and its synthesis. So far, there is a pH gradient and, therefore, while moving electrons along the carrier chains in the photo systems, the synthesis of ATP will occur. It is estimated that for every two electrons that passed through the ETC inside the thylacoid, four protons accumulate, and for every three proton, discharged with the participation of the conjugation factor out of the membrane, one ATP molecule is synthesized.
Thus, as a result of the light phase, due to the energy of the light, ATP and the NAPFN 2 are formed, used in the dark phase, and the product of water photo and water is released into the atmosphere. The total equation of the light phase of photosynthesis can be expressed as follows:
2N 2 O + 2NadFF + 2 ADF + 2N 3 PO 4 → 2 NAPFN 2 + 2 ATP + O 2
Photosynthesis is a combination of the processes of formation of light energy into the energy of chemical bonds of organic substances with the participation of photosynthetic coloring substances.
This type of nutrition is characteristic of plants, prokaryotes and certain types of single-cell eukaryotes.
With natural synthesis, carbon and water in interaction with light are converted into glucose and free oxygen:
6CO2 + 6H2O + Light Energy → C6H12O6 + 6O2
Modern plant physiology under the concept of photosynthesis understands a photometrophic function, which is a set of processes of absorption, transformation and use of luminous energy quanta in different non-repproductive reactions, including the conversion of carbon dioxide to the organic.
Phase
Photosynthesis in plants occurs in the leaves through chloroplasts - semi-autonomous two-grained organelles related to class class. With a flat form of sheet plates, high-quality absorption and full use of light energy and carbon dioxide are provided. Water necessary for natural synthesis comes from roots through water tissue. Gas exchange occurs with the help of diffusion through the dysstrian and partly through the cuticle.
 Chloroplasts are filled with colorless stroma and permeated with lamellas, which when connected to each other, form thylacides. It is in them and photosynthesis occurs. Cyanobacteria are chloroplasts themselves, therefore the apparatus for natural synthesis in them is not highlighted in separate organella.
Chloroplasts are filled with colorless stroma and permeated with lamellas, which when connected to each other, form thylacides. It is in them and photosynthesis occurs. Cyanobacteria are chloroplasts themselves, therefore the apparatus for natural synthesis in them is not highlighted in separate organella.
Photosynthesis proceeds with the participation of pigmentswhich are usually served chlorophylls. Some organisms contain another pigment - carotenoid or ficobilin. Prokaryotes have a pigment bacterio-chlorophyll, and these organisms do not excrete oxygen at the end of natural synthesis.
Photosynthesis takes place two phases - light and dark. Each of them is characterized by certain reactions and interacting substances. Consider a Read more photosynthesis phase process.
Light
 The first phase of photosynthesis It is characterized by the formation of high-energy products that are ATP, cellular source of energy, and NADF, reducing agent. At the end of the stage, oxygen is formed as a by-product. The light stage occurs necessarily with sunlight.
The first phase of photosynthesis It is characterized by the formation of high-energy products that are ATP, cellular source of energy, and NADF, reducing agent. At the end of the stage, oxygen is formed as a by-product. The light stage occurs necessarily with sunlight.
The photosynthesis process takes place in tylakoid membranes with the participation of electron carriers, ATP-synthetase and chlorophyll (or other pigment).
The functioning of electrochemical chains, according to which electron transmission and partially hydrogen protons occurs, is formed in complex complexes formed by pigments and enzymes.
Light phase process description:
- In case of sunlight on the leaf plates of plant organisms, the electron of chlorophyll in the structure of the plates occurs;
- In the active state, the particles come out of the pigment molecule and fall on the outer side of the thylacide, charged negatively. This happens simultaneously with the oxidation and subsequent restoration of the chlorophyll molecules, which select the next electrons from the water entered into the leaves;
- Then there is a photo and for the formation of ions, which give electrons and are converted to OH radicals that can participate in reactions and in the future;
- Then these radicals are connected, forming water molecules and free oxygen, entering the atmosphere;
- The thylacoid membrane acquires a positive charge due to the hydrogen ion, and on the other - negative at the expense of electrons;
- With the achievement of a 200 mV difference between the sides of the membrane, the protons pass through the ATP-synthetase enzyme, which leads to the transformation of ADP in ATP (phosphorylation process);
- With nuclear hydrogen released from water, the NADF + is restored in NADF · H2;
Whereas free oxygen in the reaction process enters the atmosphere, ATP and NADF · H2 are involved in the dark phase of natural synthesis.
Norma
 Mandatory component for this stage - carbon dioxidewhich plants are constantly absorbed from the external environment through the dust in the leaves. The processes of the dark phase are held in the stroma of chloroplast. Since at this stage is not required a lot of solar energy and will be sufficiently obtained during the light phase ATP and NADF · H2, the reactions in the organisms can occur during the day, and at night. The processes at this stage occur faster than on the previous one.
Mandatory component for this stage - carbon dioxidewhich plants are constantly absorbed from the external environment through the dust in the leaves. The processes of the dark phase are held in the stroma of chloroplast. Since at this stage is not required a lot of solar energy and will be sufficiently obtained during the light phase ATP and NADF · H2, the reactions in the organisms can occur during the day, and at night. The processes at this stage occur faster than on the previous one.
The combination of all processes occurring in the dark phase is represented as a peculiar chain of consecutive carbon dioxide transformations received from the external environment:
- The first reaction in such a chain is the fixation of carbon dioxide. The presence of the enzyme ribf carboxylase contributes to the rapid and smooth flow of the reaction, which results in the formation of a hexagonal compound, disintegrating on 2 phosphoglycerolic acid molecules;
- Then there is a rather complex cycle, including another number of reactions, upon completion of which phosphoglycerin acid is converted into natural sugar - glucose. This process is called Calvin Cycle;
Together with sugar, fatty acids, amino acids, glycerol and nucleotides are also occurring.
The essence of photosynthesis
 From the table of comparisons of light and dark phases of natural synthesis, you can briefly describe the essence of each of them. The light phase occurs in chloroplast marins with mandatory inclusion in the reaction of light energy. In reactions, such components are involved as proteins that carry electrons, ATP synthetase and chlorophyll, which, when interacting with water, form free oxygen, ATP and NAPF · H2. For the dark phase occurring in the stroma of chloroplast, the sunlight is not mandatory. The Natural Sugar (glucose) formed at the last stage of ATP and NAPF · H2 in the interaction with carbon dioxide.
From the table of comparisons of light and dark phases of natural synthesis, you can briefly describe the essence of each of them. The light phase occurs in chloroplast marins with mandatory inclusion in the reaction of light energy. In reactions, such components are involved as proteins that carry electrons, ATP synthetase and chlorophyll, which, when interacting with water, form free oxygen, ATP and NAPF · H2. For the dark phase occurring in the stroma of chloroplast, the sunlight is not mandatory. The Natural Sugar (glucose) formed at the last stage of ATP and NAPF · H2 in the interaction with carbon dioxide.
As can be seen from the foregoing, photosynthesis appears quite complex and multi-stage phenomenon, including a plurality of reactions in which different substances are involved. As a result of natural synthesis, oxygen is obtained, which is necessary for the respiration of living organisms and protect them from ultraviolet radiation by the formation of the ozone layer.
Question 1. How much glucose synthesized during photosynthesis is accounted for each of the 4 billion inhabitants of the Earth per year?
If we consider that for the year, the entire vegetation of the planet produces about 130,000 million tons of sugars, then by one resident of the Earth (provided that the population of the Earth is 4 billion inhabitants) they account for 32.5 million tons (130000/4 \u003d 32.5) .
Question 2. Where does oxygen be taken from the process of photosynthesis?
Oxygen entered into the atmosphere in the process of photosynthesis is formed during the reaction of photolysis - the decomposition of water under the action of solar light energy (2N 2 O + Energy of light \u003d 2H 2 + O 2).
Question 3. What is the meaning of the light phase of photosynthesis; Dark phase?
Photosynthesis- This is the process of synthesizing organic substances from inorganic under the action of solar light energy.
Photosynthesis in vegetable cells goes in chloroplasts. Total formula:
6Co 2 + 6N 2 O + Light energy \u003d C 6 H 12 O 6 + 6O 2.
The light phase of photosynthesis is only in the light: the quantum of light knocks the electron from the chlorophyll molecule lying in the thylacoid membrane.; An embossed electron either returns back, or enters the chain of oxidizing each other enzymes. The enzyme circuit transmits an electron to the outer side of the thylacoid membrane to the carrier of electrons. The membrane charges negatively from the outside. A positively charged chlorophyll molecule, lying in the center of the membrane, oxidizes enzymes containing manganese ions lying on the inside of the membrane. These enzymes are plotted in the reactions of photolysis of water, as a result of which N + is formed; The hydrogen protons are thrown into the inner surface of the thylacide membrane, and a positive charge appears on this surface. When the potential difference on the Tylakoid membrane reaches 200 mV, protons begin to slip through the ATP synthetase channel. ATP is synthesized.
In the dark phase from CO 2 and atomic hydrogen associated with carriers, glucose is synthesized due to ATP energy. The glucose synthesis is in the stroma of chloroplasts on enzyme systems. Total dark stage response:
6SO 2 + 24H \u003d C 6 H 12 O 6 + 6N 2 O.
Photosynthesis is very productive, but the chloroplasts of the sheet are captured to participate in this process of only 1 quantum of light out of 10,000. However, it is sufficient to ensure that the green plant can synthesize 1 g of glucose per hour from the surface of leaves with an area of \u200b\u200b1m 2.
Question 4. Why is the presence in the soil of chemosynthetic bacteria necessary for higher plants?
Plants are needed for normal growth and development of mineral salts containing elements such as nitrogen, phosphorus, potassium. Many types of bacteria that can synthesize the organic compounds they need from inorganic due to the energy of chemical oxidation reactions occurring in the cell belong to chemotrofam. Captured bacteria substances are oxidized, and the resulting energy is used on the synthesis of complex organic molecules from CO 2 and H 2 O. This process is called chemosynthesis.
The most important group of chemosynthetic organisms are nitrifying bacteria. Exploring them, S.N. Vinogradsky in 1887 opened the process chemosynthesis. Nitrifying bacteria, upholstered in the soil, oxidize ammonia, formed when rotting organic residues, to nitrogenous acid:
2MN 3 + ZO 2 \u003d 2NO 2 + 2N 2 O + 635 kJ.
Then the bacteria of other types of this group are oxidized by nitroxy acid to nitric:
2NO 2 + O 2 \u003d 2NNO 3 + 151.1 kJ.
Interacting with soil mineral substances, nitrogenous and nitric acids form salts that are the most important components of the mineral nutrition of higher plants. Under the action of other types of bacteria in the soil, the formation of phosphates, also used by higher plants.
In this way, Chemosynthesis
- This is the process of the synthesis of organic substances from the inorganic energy due to the energy of the chemical oxidation reactions occurring in the cell.