What formula is called gross formula. Structural formulas differ from molecular (gross) formulas
The gross formula of the substance I converted it to Toluene indicate that it is methylcyclohexadien. It is capable of attaching a yalene anhydride that is characteristic of conjugate dienes.
The gross formula of the substance is reliably determined only by the combination of elemental analysis with the determination of molecular weight.
The determination of the gross formula of the substance requires, thus, the analysis of homologous series of fragmentation ions and characteristic differences.
How the gross formula of the substance is established.
In addition to the spectrum of the PMR and gross formulas for the establishment of a structural formula, there are data on its nature or origin, without which the unambiguous interpretation of the spectrum would be impossible.
At the beginning of each article, the gross formula of the substance is given, its name and the structural formula is given. The search for the required substance in the directory is made according to a well-known gross formula and a formal pointer or a known name and an alphabetic pointer located at the end of the reference book.
In the first column of all tables, the gross formula of the substance is given, in the following column - its chemical formula. Then the temperature at which measurements were performed. For halogens (except iodine), only data obtained with a standard liquid nitrogen temperature (77 K) is given for other temperatures in the absence of measurements at 77 k, which is negotiated in the notes.
Mass spectrometry methods are used to identify substances, determining gross formulas of substances and their chemical structure. Important for chemistry are such physical characteristics such as the potential of ionization and the energy of the chemical is connected.
To find a compound in a formula indicator, it is necessary to pre-calculate the gross formula of the substance and lay elements on the Hill system: for inorganic substances in alphabetical order, for example H3O4R (phosphoric acid), CuO4S (copper sulfate), O7P2ZN2 (zinc pyrophosphate), etc. .
To find a compound in a formula indicator, it is necessary to pre-calculate the gross formula of the substance and lay the elements on the Hill system: for inorganic substances in alphabetical order, for example, NZO4R (phosphoric acid), CuO4S (copper sulfate), O7P2ZN2 (zinc pyrophosphate), etc. .
The possibilities of low-resolution mass spectrometry do not allow to divide the second and third stage of group identification, and the determination of the gross formula of the substance is carried out simultaneously with the restriction of the number of possible options for its attribution to specific homologous rows. By definition, the homologous group combines the series of compounds, the mass numbers of which are comparable to module 14, including isobaric. In some cases, the isobaric compounds of different rows have similar patterns of fragmentation, which manifests itself in the similarity of their low-resolution mass spectra.
The mass of molecular ion (180,1616) is measured with high accuracy, which makes it possible to immediately determine the gross formula of the substance.
Based on the above, in an elemental analysis of organic compounds proposed branding methods for determining the stoichiometry of molecules characterizing the gross formula of the substance. Basically, these methods are designed to clarify the stoichiometry of organogen elements: carbon, hydrogen and nitrogen. They are based on comparing analytical signals of product-mineralization of the substance. As such signals, such signals are, for example, the area of \u200b\u200bchromatographic peaks, the volumes of titrant, common to two elements, and others. Thus, work without weights with micro and ultramicrocolism is possible.
Quantitative analysis of polymers includes the following questions: 1) a quantitative elemental analysis that allows you to establish a gross formula of substance; 2) determining the number of functional and end groups in polymer chains; 3) the definition of the mall.
The exact values \u200b\u200bof molecular weight can be obtained from the mass spectra and are based on a certain alternative assumptions about the gross formula of the substance, its qualitative and quantitative compositions. Thus, in particular, the odd amount of molecular weight can serve as proof of the presence in the molecule of one (three, five, in general - odd number) of the nitrogen atom: nitrogen is the only element-organogen with an odd valence at an even ATO. In contrast, even molecular weight indicates the absence of nitrogen or the possibility of the even number of its atoms. Thus, for example, organic substance with M 68 can have only three gross formulas: CSHS, 4 6 or SZN, and the accounting will significantly facilitate the interpretation of spectral data and the final choice of structure.
An even more valuable source of the necessary additional information is the data of quantitative (elemental) analysis, which, in combination with the definition of molecular weight, is allowed to establish a gross formula of the substance.
An even more valuable source of the necessary additional information is the data of quantitative (elemental) analysis, co: those in combination with the molecular weight determination allow you to establish a gross formula of substance. Classic (chemical) Methods for establishing a gross formula are now increasingly replaced by mass-rometric mass-based, based on the accurate measurement of the intensity of isotopic lines of molecular ions or a very accurate measurement of mass numbers on high-resolution spectrometers.
An even more valuable source of the necessary additional information is the data of quantitative (elemental) analysis, which, in combination with the determination of molecular weight, make it possible to establish the gross formula of the substance.
Notice, this is a rare case when the gross formula is responsible to one substance. Usually, on the basis of these data, we can only indicate the gross formula of substance, but not a structural formula. And often we can not even relate a substance with a specific class. To obtain the structural formula of the substance, additional data on the chemical properties of this substance are necessary.
Elemental analysis is used to quantify organic and elementanganic compounds containing nitrogen, halogen, sulfur, as well as arsenic, bismuth, mercury, antimony and other elements. An elemental analysis can also be applied to qualitatively confirming the presence of these elements in the compounds under study or to establish or confirm the gross formula of the substance.
The last row is less likely, since its sign is the presence in the spectra of intensive peaks of the 4th homologous group, which in the case under consideration. Subsequent detailing of the classification can be unambiguously carried out by spectra of ion series (see section 5.5), however, given the high intensity of peaks of molecular ions in this spectrum, it is advisable to clarify the gross formula of the substance using isotopic signals.
The concept of homology is one of the most important in organic chemistry, and homologous ranks constitute the basis of the modern classification of organic compounds. Issues of accessories of compounds to different homologous rows are very important and are associated, for example, with the problems of isomerism in organic chemistry, in particular with the creation of effective algorithms for determining the number of possible isomers on the gross formula of a substance using a computer.
Schemes for quantitative elemental analysis. In elemental analysis, there is a tendency to reduce manual labor and an increase in the accuracy of definitions. The development of the instrument equipment has allowed the device for an automatic elemental analysis in the very last years, in which carbon dioxide, water and nitrogen sample, which formed when burning the sample, is sent to the gaseous chromatograph attached to the device, with which their simultaneous quantitative determination is carried out. On the other hand, the use of a mass spectrometer, high resolution (see section 1.1.9.3) makes it possible to determine the gross formula of the substance without the conduct of the number of elemental analysis.
Developed a dialog mode of operation of the Raster system. The exchange of information between man and computer is carried out through the alphanumeric display. The program produces a survey of the working, simultaneously indicating the form of the answer. Information on the types of experimental spectra that are available in the presence of their signs and spectral parameters is required. After entering the entire spectral information and gross formulas of the substance, the operator indicates the mode of constructing implications - logical relations between the signs of the spectrum and the structure of the compound. The operator has the ability to make any changes to them: to exclude or add information to library fragments, remove any implications or add new ones. As a result of the solution of the system of agreed logic equations on the display, sets of fragments that satisfy spectra and chemical information are issued.
When processing the mass spectra manually, the necessary identification stage is to determine the class of substance. This stage in the apparent or implicit form is also included in many complex identification algorithms intended for computers. Such an operation can be done in the case when the mass spectrum of the material determined has not been previously known, but the regularities of the fragmentation of the combination of the compounds to which it applies is well studied. This is possible on the basis of the qualitative and quantitative patterns of fragmentation common for this class or homologous series. If the unknown component managed to register so important to identify the peak, as a peak of a molecular ion, then, in combination with information about the class of compound, the molecular weight allows the brutate formula of the substance. It should be noted that the use of isotopic peaks to determine the gross formula with chromato mass spectrometric analysis has a limited value and is possible only with high intensity of these peaks and peak of the molecular ion. For individual groups of isomers of aromatic and paraffin hydrocarbons, individual identification algorithms have been developed, based on some quantitative characteristics of their mass spectra.


 Molecular, or gross formula, shows which atoms and in what quantity are included in the molecule, for example, from 6 H 6 CH 4 ° C 2 H 3 CL. O The molecular formula does not reflect the structure of the molecule, the structural formula should reflect: the nature of atoms, which are part of the molecule, their number and the sequence of their connection between themselves, as well as the type of communication between atoms.
Molecular, or gross formula, shows which atoms and in what quantity are included in the molecule, for example, from 6 H 6 CH 4 ° C 2 H 3 CL. O The molecular formula does not reflect the structure of the molecule, the structural formula should reflect: the nature of atoms, which are part of the molecule, their number and the sequence of their connection between themselves, as well as the type of communication between atoms.

 Hydrocarbons with four carbon atoms may have a carbon skeleton of a branched, unbranched or cyclic structure: atoms in the molecule can be connected by single, double or triple connections:
Hydrocarbons with four carbon atoms may have a carbon skeleton of a branched, unbranched or cyclic structure: atoms in the molecule can be connected by single, double or triple connections:
 Electronic and structural formulas of molecules do not reflect the spatial structure of molecules. Atomic orbital models of molecules Simple line (valence damn) depicts the axis of orbitals lying in the plane of the pattern; The solid wedge corresponds to AO located above the plane of the pattern; The shaded wedge depicts AO directed for this plane.
Electronic and structural formulas of molecules do not reflect the spatial structure of molecules. Atomic orbital models of molecules Simple line (valence damn) depicts the axis of orbitals lying in the plane of the pattern; The solid wedge corresponds to AO located above the plane of the pattern; The shaded wedge depicts AO directed for this plane.



 The essence of this process is to break the chemical bonds in the initial substances and the formation of new links in reaction products. Organic reactions are recorded not in the form of equations, but in the form of reaction schemes, in which attention is paid not so much with the stoichiometric ratio of reagents, how many reaction conditions. In these schemes, the initial products (reagents) are separated from the reaction products with an arrow, over which the reaction conditions and catalysts indicate, and under the arrow with a "minus" sign - the compounds that are formed during the reaction
The essence of this process is to break the chemical bonds in the initial substances and the formation of new links in reaction products. Organic reactions are recorded not in the form of equations, but in the form of reaction schemes, in which attention is paid not so much with the stoichiometric ratio of reagents, how many reaction conditions. In these schemes, the initial products (reagents) are separated from the reaction products with an arrow, over which the reaction conditions and catalysts indicate, and under the arrow with a "minus" sign - the compounds that are formed during the reaction


 Reactions of decomposition: As a result of the decomposition reaction, several less complex or simple substances are formed from the molecule: the splitting of the carbon skeleton of large molecules during heating and in the presence of catalysts (decomposition reaction at high temperature is called pyrolysis) a low molecular weight molecule is cleaved by two adjacent s-atoms (increase in multiplicity of communication) or from other atoms to form a cycle
Reactions of decomposition: As a result of the decomposition reaction, several less complex or simple substances are formed from the molecule: the splitting of the carbon skeleton of large molecules during heating and in the presence of catalysts (decomposition reaction at high temperature is called pyrolysis) a low molecular weight molecule is cleaved by two adjacent s-atoms (increase in multiplicity of communication) or from other atoms to form a cycle
 The formation of two new bonds in the reactant molecule occurs. At the same time, the multiplicity of the coupling of the reactant decreases. An atom group or a group of atoms is replaced by another atom or group of atoms: the starting material and the reaction product are isomers (structural or spatial).
The formation of two new bonds in the reactant molecule occurs. At the same time, the multiplicity of the coupling of the reactant decreases. An atom group or a group of atoms is replaced by another atom or group of atoms: the starting material and the reaction product are isomers (structural or spatial).
 Classification of reactions in the direction of the chemical reaction, which, with some and the same conditions, can go directly in the opposite directions. When leveling the rates of direct and reverse reactions (condition of chemical equilibrium), the reversible reaction ends. It goes almost to the end in one direction.
Classification of reactions in the direction of the chemical reaction, which, with some and the same conditions, can go directly in the opposite directions. When leveling the rates of direct and reverse reactions (condition of chemical equilibrium), the reversible reaction ends. It goes almost to the end in one direction.



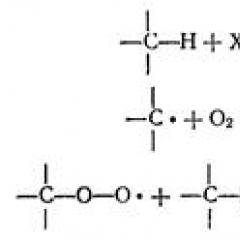
 The conditions for conducting radical reactions: Increased temperature (often the reaction is carried out in the gas phase), the effect of light or radioactive radiation, non-polar solvents, the presence of compounds - sources of free radicals (initiators) of the reaction with the participation of free radicals is characteristic of compounds with non-polar and weaklyolar bonds. Such links (for example, C-C, C-H, CL-CL, O-O, etc.) are prone to a gomolitical break
The conditions for conducting radical reactions: Increased temperature (often the reaction is carried out in the gas phase), the effect of light or radioactive radiation, non-polar solvents, the presence of compounds - sources of free radicals (initiators) of the reaction with the participation of free radicals is characteristic of compounds with non-polar and weaklyolar bonds. Such links (for example, C-C, C-H, CL-CL, O-O, etc.) are prone to a gomolitical break
 Heterolithic reactions (ionic) overall reaction scheme: CH 3) 3 C Cl + H 2 O (CH 3) 3 C-OH + HCl process stages
Heterolithic reactions (ionic) overall reaction scheme: CH 3) 3 C Cl + H 2 O (CH 3) 3 C-OH + HCl process stages
 Conditions for conducting ionic reactions: low temperature; Polar solvents capable of solvation of the generated ions. Such reactions are characteristic of compounds with polar bonds (C-O, C-N, C-CL) and connections with high polarizability (C \u003d C, C \u003d C - C \u003d C, C \u003d O, etc.). Than a polar connection, the easier it is broken by the Jonian mechanism !!!
Conditions for conducting ionic reactions: low temperature; Polar solvents capable of solvation of the generated ions. Such reactions are characteristic of compounds with polar bonds (C-O, C-N, C-CL) and connections with high polarizability (C \u003d C, C \u003d C - C \u003d C, C \u003d O, etc.). Than a polar connection, the easier it is broken by the Jonian mechanism !!!










 In 1815, the French chemist Bio opened a new kind of isomerism optical, or a mirror. He found that some. Organic substances in a liquid or dissolved state rotate the plane of polarized light.
In 1815, the French chemist Bio opened a new kind of isomerism optical, or a mirror. He found that some. Organic substances in a liquid or dissolved state rotate the plane of polarized light.
 Compounds that change (rotate) polarization plane are called optically active, they exist in two optical isomers. , And one of them rotates the polarization plane to the right, and the other is at the same angle, but to the left. To denote these rotations, use signs (+) and (-), which put in front of the optical isomer formula. All optically active substances contain in their molecules at least one asymmetric carbon atom (in the formulas, this atom is indicated by an asterisk), i.e. carbon, which is associated with four different atoms or groups of atoms
Compounds that change (rotate) polarization plane are called optically active, they exist in two optical isomers. , And one of them rotates the polarization plane to the right, and the other is at the same angle, but to the left. To denote these rotations, use signs (+) and (-), which put in front of the optical isomer formula. All optically active substances contain in their molecules at least one asymmetric carbon atom (in the formulas, this atom is indicated by an asterisk), i.e. carbon, which is associated with four different atoms or groups of atoms
 Any organic compound containing an asymmetric carbon atom can be represented as two spatial forms (models), which when applied in space cannot be combined with each other. These two forms (models) differ from each other as a subject from their mirror image. Therefore, such a isomeria was called "mirror". Mirror Optical Isomers Butanol-2
Any organic compound containing an asymmetric carbon atom can be represented as two spatial forms (models), which when applied in space cannot be combined with each other. These two forms (models) differ from each other as a subject from their mirror image. Therefore, such a isomeria was called "mirror". Mirror Optical Isomers Butanol-2
 Molecules (or their models), which cannot be combined in space (when applied) and which belong to each other as an item to their mirror image, is called chiral (from Greek. Hearos - hand, hands-made). An example can serve as the right and left, which are not combined when applied. Thus, the optical isomerism is a phenomenon caused by chirality.
Molecules (or their models), which cannot be combined in space (when applied) and which belong to each other as an item to their mirror image, is called chiral (from Greek. Hearos - hand, hands-made). An example can serve as the right and left, which are not combined when applied. Thus, the optical isomerism is a phenomenon caused by chirality.
 As an image of optically active substances on paper use projection formulas proposed by E. Fisher. Formula Fisher
As an image of optically active substances on paper use projection formulas proposed by E. Fisher. Formula Fisher

 It was generally approved that optically active compounds in which hydroxyl in the projection formula is located to the right of an asymmetric carbon atom, refers to D-row, and on the left - to L-row. As such a standard, glycerin aldehyde D (+) - glycerin aldehyde L (-) - glycerin aldehyde
It was generally approved that optically active compounds in which hydroxyl in the projection formula is located to the right of an asymmetric carbon atom, refers to D-row, and on the left - to L-row. As such a standard, glycerin aldehyde D (+) - glycerin aldehyde L (-) - glycerin aldehyde
 Conformational isomeria with internal rotation of groups of atoms around simple links arise various spatial structures, called conformations. These movements do not disturb the structure of molecules. Internal rotation around the links C-H cannot change the spatial orientation of atoms in molecules (therefore, various conformations of methane molecule) are not arisen). However, rotation around the connection C-C in the ethan molecule leads to a huge set of conformation. The most important and most different from each other are called obsolete and inhibited conformations. Conformations are depicted both spatial and projection formulas. This uses the so-called projection of Newman: the molecule is oriented in such a way that the connection around which rotation occurs, is projected into the center of the circle, and the connection from the atom closeer is depicted by lines emanating from the center of the circle, and the bonds coming from the remote atom are drawn lines outside the circle.
Conformational isomeria with internal rotation of groups of atoms around simple links arise various spatial structures, called conformations. These movements do not disturb the structure of molecules. Internal rotation around the links C-H cannot change the spatial orientation of atoms in molecules (therefore, various conformations of methane molecule) are not arisen). However, rotation around the connection C-C in the ethan molecule leads to a huge set of conformation. The most important and most different from each other are called obsolete and inhibited conformations. Conformations are depicted both spatial and projection formulas. This uses the so-called projection of Newman: the molecule is oriented in such a way that the connection around which rotation occurs, is projected into the center of the circle, and the connection from the atom closeer is depicted by lines emanating from the center of the circle, and the bonds coming from the remote atom are drawn lines outside the circle.


Gross, structural and electronic compound formulas
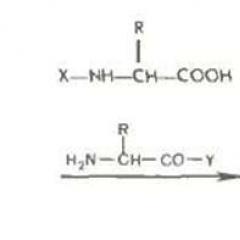
The second postulate of Worthler. The chemical reactivity of certain groups of atoms is significantly dependent on their chemical environment, that is, with which atoms or groups of atoms are adjacent to a certain group.
The formulas of the compounds we used in the study of inorganic chemistry reflect only the number of atoms of a particular element in the molecule. Such formulas are called "gross formulas", or "molecular formulas".
As it follows from the first postulate of Wouthler, not only the number of certain atoms in the molecule is important in organic chemistry, and the order of their binding, that is, gross formulas are not always appropriate to use for organic compounds. For example, for clarity when considering the structure of methane molecule, we used structural formulas - a schematic representation of the order of binding of atoms into the molecule. In the image of structural formulas, the chemical bond is denoted by a feature, a double bond - two invasses, etc.
The electronic formula (or Lewis formula) is very similar to the structural formula, but in this case depicts are depicted non-formed bonds, and electrons, like those that form a bond and those that do not form it.
For example, the sulfate acid already considered can be written using the following formulas. Gross formula - H 2 80 4, structural and electronic formulas have this kind:
Structural formulas organic compounds
Almost all organic substances consist of molecules, the composition of which is expressed by chemical formulas, for example CH 4, C 4 H 10, C 2N 4 O 2. And what structure have organic molecules? This question was asked in the middle of the XIX century the founders of organic chemistry - F. Kekule and A. M. Vuteler. Exploring the composition and properties of various organic substances, they came to the following conclusions:
Atoms in organic substances molecules are connected by chemical bonds in a certain sequence, according to their valence. This sequence is customary to be called a chemical structure;
Carbon atoms in all organic compounds are ready, and other elements show valence characteristic of them.
This provision is the basis of the theory of the structure of organic compounds formulated by O. M. Butlerov in 1861.
The chemical structure of organic compounds is clearly supplied with structural formulas in which chemical bonds between atoms are denoted by dashes. The total number of drops derived from the symbol of each element is equal to its valence of an atom. Multiple communication is depicted in two or three invasses.
Using the example of a saturated hydrocarbon propane C 3 H 8, we consider how to draw up the structural formula of the organic matter.
1. We depict carbon skeleton. In this case, the chain consists of three carbon atoms:
C-S- FROM
2. Carbon tetravalent, therefore, from each carbon atom, we depict insufficient traits so that four features are next to each atom:

3. Finish the symbols of hydrogen atoms:

Often, structural formulas are written in abbreviated form, without depicting the connection of C - N. Abbreviated structural formulas much more compact than the deployed:
CH 3 - CH 2 - CH 3.
The structural formulas show only the sequence of the compound of atoms, but do not reflect the spatial structure of molecules, in particular, valence angles. It is known, for example, that the angle between connections C in propane is 109.5 °. However, the structural formula of propane looks like this angle is 180 °. Therefore, it would be more correct to record the structural formula of propane in a less convenient, but more true:
Professional chemists use the following structural formulas, in which neither carbon atoms nor hydrogen atoms are not shown at all, but only the carbon skeleton is depicted in the form of a combined C-C-links, as well as functional groups. In order for the backbone does not look like one solid line, chemical connections are depicted at an angle to each other. Thus, in the propane molecule with 3N 8 only two connections C-C, so propane is depicted by two dashes.
Homological rows of organic compounds
Consider the structural formulas of two compounds of one class, such as alcohols:

MEKLE MEETLOGO CH 3 It and ethyl C 2 H 5 It has the same functional group, it is common to the whole class of alcohols, but differ in the carbon skeleton length: in ethanol one carbon atom. Comparing the structural formulas, it can be noted that with an increase in the carbon chain to one carbon atom, the composition of the substance changes to the CH group 2, with the lengthening of the carbon chain by two atoms - into two groups of CH 2.
Compounds of one class having a similar structure, but differing in composition on one or more groups of CH 2, called homologues.
The CH 2 group is called homologous difference. The combination of all homologues forms a homologous row. Methanol and ethanol belong to the homologous row of alcohols. All substances of one row have similar chemical properties, and their composition can be expressed by the general formula. For example, the general formula of the homologous series of alcohols - with n 2 n +1 won, where n - natural number.
|
Class of connections |
General formula |
General formula with the allocation of the functional group |
|
Alkana |
C n 2 n + 2 |
|
|
Cycloalkani |
C n 2 n |
|
|
Alkenes |
C n 2 n |
|
|
Alkadin |
With N H 2 N-2 |
|
|
Alkini |
With N H 2 N-2 |
|
|
Single-core arena (Gomological row of benzen) |
C n 2 n-6 |
|
|
Singo-alcohol nasinenі |
With N 2 n + 2 in |
C n 2 n +1 in H |
|
Multiatomic alcohols |
With n 2 n + 2 o x |
With n 2 n + 2-x (in h) x |
|
Aldehydes |
C n 2 n in |
With n 2 n +1 cho |
|
Simple carboxylic acids |
With NN 2 N O 2 |
C n 2 n +1 COOH |
|
Estheri. |
C n 2 n in |
With N H 2 n +1 COOC N H 2N + 1 |
|
Carbohydrates |
With N (H 2 O) M |
|
|
Amines primary |
With n 2 n + 3 n |
C n 2 n +1 NH 2 |
|
Amino acids |
With n 2 n +1 no |
H 2 NC N H 2N COOH |
download
Abstract on the topic:
Chemical formula
Chemical formula - Reflection of information on the composition and structure of substances using chemical signs, numbers and separating signs - brackets.
The composition of complex substances molecules is expressed with the help of chemical formulas.
On the basis of the chemical formula, the name of the substance can be given.
Chemical formula Indicates:
- 1 molecule or 1 mol of substance;
- qualitative composition (from which chemical elements a substance consists);
- quantitative composition (how many atoms of each element contains a substance molecule).
- The formula H N O 3 is:
- nitric acid;
- 1 nitric acid molecule or 1 mole of nitric acid;
- qualitative composition: a nitric acid molecule consists of hydrogen, nitrogen and oxygen;
- quantitative composition: The composition of the nitric acid molecule includes one atom of the hydrogen element, one atom of the nitrogen element, three atoms of the oxygen element.
Views
Currently distinguish the following types of chemical formulas:
- Simplest formula. It can be obtained by experimentally through the determination of the ratio of chemical elements in a substance using the values \u200b\u200bof the atomic mass of the elements. Thus, the simplest formula of water will be H 2 O, and the simplest formula of benzene ch (in contrast to C 6 H 6 - true, see below). Atoms in the formulas are indicated by the signs of chemical elements, and their relative number - numbers in the format of the lower indices.
- True formula. It can be obtained if the molecular weight of the substance is known. The true formula of water H 2 O, which coincides with the simplest. The true formula of benzene with 6 H 6, which is different from the simplest. True formulas are also called gross formulas or empirical. They reflect the composition, but not the structure of the molecules of the substance. The true formula shows the exact number of atoms of each element in the same molecule. This quantity corresponds to the index - a small number after the symbol of the corresponding element. If the index is 1, that is, there is only one atom of this element in the molecule, this index does not indicate.
- Rational formula. In the rational formulas, groups of atoms characteristic of classes of chemical compounds are distinguished. For example, a group is allocated for alcohols. When recording a rational formula, such groups of atoms consist in parentheses (OH). The number of repetitive groups is indicated by the numbers in the format of the lower indices that are put immediately behind the closing bracket. Square brackets are used to reflect the structure of complex compounds. For example, K 4 is a potassium hexationanocobeat. Rational formulas are often found in a half-hearted form, when a part of the same atoms is shown separately for better reflection of the structure of the substance molecule.
- Structural formula. In graphical form shows the relative arrangement of atoms in the molecule. Chemical bonds between atoms are denoted by lines. There are two-dimensional (2D) and three-dimensional (3D) formulas. Two-dimensional are the reflection of the structure of the substance on the plane. Three-dimensional allow the most close to theoretical models of the structure of the substance to represent its composition, the relative position, connection and distances between atoms.
- Ethanol.
- The simplest formula with 2 H 6 o
- True, empirical, or gross formula: C 2 H 6
- Rational formula: from 2N 5
- Rational formula in half-round form: CH 3 CH 2
- Structural formula (2D):
There are other ways to write chemical formulas. New ways appeared in the late 1980s with the development of personal computer equipment (Smiles, WLN, ROSDAL, SLN, etc.). In personal computers to work with chemical formulas, special software are also used, called molecular editors.
Notes
- 1 2 3 Basic concepts of chemistry - de.gubkin.ru/chemistry/ch1-th/node6.html
This abstract is based on an article from Russian Wikipedia. Synchronization executed 07/10/11 17:38:37
Related Schedules: