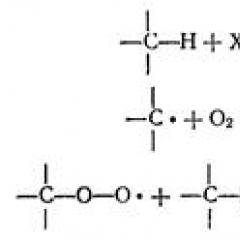
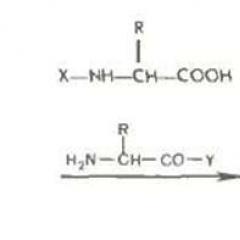
The solid phase synthesis of peptides with protective groups of VOS and FMOC. The structure of peptides solid-phase synthesis of cardioactive peptide
Peptide communication has the properties of partially dual communication. This is manifested in reducing the length of this connection (0.132 nm) compared with the length of the simple bond with N (0.147 nm). A partially bobbling nature of the peptide communications makes it impossible free rotation of the substituents around it, so the peptide group is flat and has usually trans-configuration (F-La I). In order, the axle of the peptide chain is a series of hard planes with movable ("hinged") articulation in a place where the asymmetric atoms with (in F-le i are designated by an asterisk).
In peptide solutions there is a preferred formation of certain conformers. With the lengthening of the chain, more pronounced stability is acquired (similar to proteins) ordered elements of the secondary structure. The formation of the secondary structure is especially characteristic of regular peptides, in particular for polyamine acids.
Properties
Oligopeptides by properties are close to amino acids, polypeptides are similar to proteins. Oligopeptides are usually crystalline substances that decompose when heated to 200,300 0 C. They are well soluble in water, diluted acids and alkalis, are almost not soluble in organic solvents. Exceptions are oligopeptides constructed from hydrophobic amino acid residues.
Oligopeptides have amphoteric properties and, depending on the acidity of the medium, can exist in the form of cations, anions or zwitter ions. The main absorption bands in the IR spectrum for the group NH 3300 and 3080 cm -1, for the group C \u003d O 1660 cm -1. In UV spectra, the absorption band of the peptide group is located in the region of 180-230 nm. The peptide isoelectric point (PI) varies widely and depends on the composition of amino acid residues in the molecule. The magnitudes of the RK and peptides are for A-coxy approx. 3, for -h 2 approx. eight.
Chemical properties of oligopeptides are determined by functional groups contained in them, as well as peptide features. Their chemical transformations are largely similar to the corresponding amino acid reactions. They give a positive buret reaction and ningidrin reaction. Dipeptides and their derivatives (especially ethers) are easily cyclized, turning into diketopiperazines. Under the action of 5.7 normal hydrochloric acid peptides are hydrolyzed to amino acids for 24h at 105 0 C.
Synthesis peptide
In peptide synthesis, known from the organic chemistry of the reaction of obtaining amides and specially developed methods for the synthesis of peptides are used. To successfully implement these synthesis, it is necessary to activate the carboxyl group, i.e. Increase electricity carbonyl carbon. This is achieved by chemical modification of the carboxyl group of amino acids. The type of such modification usually determines the name of the method of peptide synthesis.
1. Chloranhydride method.
The method is based on the reaction of obtaining amides to the interaction of acid chloride crides with the corresponding amines. This method was obtained first peptides. At present, this method is extremely rare, since it is accompanied by the formation of by-products and peptide racemic.

2. Azide method
The initial substance in this method is most often ethyl ether N-protected amino acid, from which hydrazide is obtained, the latter is obtained using sodium nitrite in the presence of hydrochloric acid in acid azide. In the reaction, hydrazine is usually used, in which one of the nitres is blocked by a protecting group (Z-carbobenzoxy or carbohytercutuclear), which avoids the formation of side dihydrazides. Azides when interacting with C-protected amino acids in mild conditions form peptides.
The racemization in this method is minimized, but there may be adverse reactions, namely: azides can be rearranged into isocyanates, which in turn, when interacting with alcohol used as a solvent, form urethanes.

3. Mixed anhydrides
Mixed amino acid anhydrides with coal acid derivatives obtained, for example, were found wide use in peptide synthesis, obtained, for example, using isobutylchloricarbonate: 
The reaction in this synthesis is carried out at a low temperature (-10 .. -20 s), quite quickly, which significantly reduces the possibility of forming by-products and racemia. The fast stepped synthesis of peptides using mixed anhydrides is called Rema-synthesis. Methods of education using mixed anhydrides are widely used in the solid-phase synthesis of peptides.
Thus, peptide synthesis requires accounting and harsh compliance with some factors. Thus, in order to reduce the formation of by-products and racemia, the following type conditions for the reaction of the formation of peptide communication are recommended:
1) the process must be carried out at low temperatures, the reaction time must be minimal;
2) the reaction mass must have a pH close to neutral;
3) organic bases are used as acid binding reagents as piperidine, morpholine, etc.;
4) The reaction is preferably in anhydrous media.
Solid-phase synthesis
Solid-phase synthesis - methodical approach to synthesis of oligomers (polymers) using solid insoluble mediarepresenting an organic or inorganic polymer.
In the early 1960s, a new approach was proposed to solve the problems of isolation and purification arising in peptide synthesis. Later by the opening of this approach, R.B. Merrifield, in his Nobel lecture told how it happened: "Once I had a thought about how the goal of a more effective synthesis of peptides was reached. The plan was to collect the peptide chain of the postal, and during the synthesis the circuit should be in one end tied to a solid carrier. " As a result, the release and purification of intermediate and target peptide derivatives was simply reduced to filtration and thorough washing of solid polymer to remove all excess reagents and by-products remaining in solution. Such a mechanical operation can be performed quantitatively, easily standardized and can even be automated. Consider this procedure in more detail.
Solid-phase synthesis,
methodich. The approach to the synthesis of oligo (poly) measures using a solid insoluble carrier (N.), which is a HARD. or noorg. polymer. That is, it is based on the fact that the first link of the future oligomer is covalently fixed on the "anchor" group N., the circuit extension is carried out by standard monomers by conventional schemes used for synthesis in the R-RAX. On making. Synthesian stage. The oligomer is cleaved from N. and is purified by the corresponding methods. T. s. Apply in the OSN. To obtain polypeptides, oligo-nucleotides and oligosaccharides.
In the synthesis of polypeptides as N. NAB. Styrene copolymer and 1-2% of divinyl benzene, modified by the administration of a dimethoxybenzyl chloride anchor group to attach the first (with a protected NH 2 group) by C-End, for example:
After removing the N-protecting group, the building of the polypeptide chain is carried out by standard methods of peptide synthesis in P-re (see. Peptides). As condensing agents is NaB. Often used or pre-convert amino acids into an activift. Ether.
In the synthesis of oligonucleotides as N. use macroporous glasses or. An anchor group serves a carboxyl group separated from N. Spets. "Foot", eg:

In purine or pyrimidic base
At the first stage, the nucleoside is attached to the carrier of 3 "-hydroxyl deoxyribose group, in K-swarm, a strong group in position 5" is protected by a dimoxyitortime group (CH 3 OS 6N 4) 2 (C 6 H 5) C (DMTR ); Number of late after its cleavage is easily measured by spectropometrically, which serves as quotations. The characteristic of the loading of the carrier and allows you to estimate the outputs at the subsequent stages of the extension of the oligonucleotide chain. After removal of the DMTR group, the circuit assembly is carried out using phosphylatidides (F-La I; M. Kapozers, 1980) or phosphonates (hydrophosphonates) (II; R. Ryiszgg, 1986):

To implement T. with. High yields are needed (at the level of 96-99%) at each stage of the Pension, as well as effective methods for cleaning and highlighting the synthesis. connections.
The use of a solid phase makes it possible to significantly simplify and speed up each stage of increasing the chain of the oligomer, since the separation of excess components, condensing agents and by-products located in P-re, is achieved by filtling the reaction. Mixtures and washing N. A suitable set of P-Rite-lei. T. OBR., The process of assembling the oligomer chain disintegrates to a number of standard operations: the release of the growing end of the chain, dosing the next protected monomer and the condensing agent, the supply of this mixture onto a column with N. for the calculated time and washing N. A suitable R-Rider. The cycle of extension of the monomer unit m. B. Automated.
At the heart of automatic. Prom. Synthesizers lies with a general schematic diagram (see Fig.). Numerous The synthesizer models differ in the design of the columns and their number, the method of feeding the reagents and R-Ryteli, etc. Control and programming are carried out using a built-in or rendered computer.

Concept device automatics. Prom. synthesizers (electric. Control line is indicated by dotted line): 1-rini of the supply of monomers (M 1, m N.) and condensing agent (ka); 2-line feeding of reagents (eg, oxidizing agents, acylating agents, kt, etc.) and phelters (P 1, R N.); 3 - switchable valves; 4-column with carrier, equipped with distribution. valve; 5-photometric. cell; 6 meter; 7-control and programming unit; 8-display.
The potential features of this is demonstrated by the synthesis A (R. Merryfield, 1969) and human growth hormone (D. Yamashiro, 1970) with a length of 124 and 183 amino acids, respectively. However, due to a small but constant racemication occurring in the formation of peptide communications, Synthesian. Possess low biol. Activity, therefore automatic. Synthesizers are used ch. arr. To obtain short polypeptides (10-30 links), including for the preparative synthesis of protein (1g).
That is, the merryphield (1962) for the synthesis of polypeptides is proposed and entered into the practice of polypeptides, and then the synthesis of oligonucleotides (R. Letzinger, 1964) and Oligosaccharides (A. Patchernik, 1973) are common.
There is another important aspect of using N. for holding MN. Org. P-Qii (, halogenation, etc.). In this case, the modified. N. acts as a polymer reagent or catalyst, and all transformations of the substrate occur in P-RE. For example, the ROP (OH) (OH) 2 phosphate (OH) 2 phosphates are carried out using a cross-linked polystyrene, modified by a sulfochloride group as a condensing agent.
LIT: Chemistry of polypeptides, per. from English, M., 1977; Polyner-Supported Reactions in Organic Synthesis, Ed. By P. Hodge, D.C. Sherrington, Chichester, 1980; Oligonucleotide Synthesis, a Practical Approach. Wash., 1984. B.k. Potapov.
Chemical encyclopedia. - M.: Soviet Encyclopedia. Ed. I. L. Knunyantsa. 1988 .
Watch what is "solid-phase synthesis" in other dictionaries:
solid-phase synthesis - combinatorial chemical synthesis synthesis of various compositions that uses solid support to separate the compositions during the synthesis, thereby simplifying the identification of the resulting compositions was attached to the growing peptide chain, while dicyclohexylcarbodiimide was used as a condensing agent (DCC with an additive of N-hydroxyuccinimide ( GS). The active peptide was separated from the polymer, the protecting groups were removed by the action all this can be written as a schema (see page 336; TFUK - trifluoroacetic acid).
The total yield of the product 85 was about 40%, from which only 10% of purified active 84 can be obtained.
The blocks were synthesized by the binding of fragments obtained either by linear or converged synthesis. When constructing a block (1-12), the binding of fragments was carried out through the proline links, since the racemization is minimal. In most cases, binding was carried out by the activated ether using pentachlorophenyl (RSR) or -Netrophenyl ethers. The synthesis scheme is a binsiloxicarbonyl prosthetic block is shown below.

In cases where the proline links cannot be used as a place of binding of fragments, their compound is carried out a azid method at which less racemization.
The total activity of the product (80%) is higher than in the case of a malcrop pedestal synthesis (30%).

Combinatorial synthesis can be carried out not only in solution (liquid-phase synthesis), but also on the surface of a solid chemically inert phase. In this case, the first starting material is chemically "sewn" to functional groups on the surface of the polymer carrier (the ester or amide bond is most often used) and is treated with a solution of the second source substance, which is taken into a significant excess to the reaction to end. In such a form of reaction, there are definite convenience, since the product is relieved: the polymer (usually in the form of granules) is simply filtered, is thoroughly washed from the second reagent residues and chemically cleaved the target compound from it.
In organic chemistry there is not a single reaction that provides in practice quantitative yield of target products anyway. The only exception is apparently the complete combustion of organic substances in oxygen at high temperatures to CO 2 and H 2 O. Therefore, the cleaning of the target product is always indispensable, and often the most difficult and time-consuming task. A particularly difficult task is to isolate the products of peptide synthesis, for example, the separation of a complex mixture of polypeptides. Therefore, it was in peptide synthesis that the synthesis method on a solid polymer substrate, developed in the early 60s of the twentieth century, was obtained the greatest distribution developed at the beginning of the 60s.
The polymer carrier in the Merryfield method is a granulated crosslinked polystyrene containing chloromethyl groups in benzene nuclei, which are linkers connecting the substrate with the first amino acid residue of the polypeptide. These groups convert the polymer into the functional analogue of benzyl chloride and reported the ability to easily form compound ester connections when reactions with carboxylate anions. Condensation of such a resin with N-protected amino acids leads to the formation of appropriate benzyl esters. The removal of n-protection from gives a C-protected derivative of the first amino acid, covalently associated with the polymer. The aminoocylation of the released amino group N-protected derivative of the second amino acid, followed by the removal of N-protection leads to a similar derivative of the dipheptide also tied to the polymer:
Such a two-stage cycle (removal of protection - aminoocylation) may be, in principle, repeated as many times as required to build a polypeptide chain of a given length.
The further development of Meriferd's ideas was directed primarily to the search and creation of new polymer materials for substrates, the development of methods for separating products and creating automated installations for the entire cycle of polypeptide synthesis
The efficiency of the Merofield method was demonstrated by the successful synthesis of a number of natural polypeptides, in particular insulin. Particularly visual advantages were demonstrated on the example of the synthesis of the enzyme ribonuclease. For example, the price of considerable effort, for several years, Hirschmen with 22 employees performed the synthesis of the ribonuclease enzyme (124 amino acid residues) with the help of traditional liquid phase methods. Almost at the same time, the same protein was obtained by automated solid phase synthesis. In the second case, the synthesis, including only 11,931 different operations, including 369 chemical reactions, was carried out by two participants (Gatt and Merryfin) in just a few months.
The ideas of the merryphorus served as the basis for creating various methods of combinatorial synthesis of libraries of polypeptides of various buildings.
So in 1982, the original strategy of the multistage parallel synthesis of peptides on the solid phase known as the "Split method" was proposed ( split. - splitting, separation) or "Mix and stripped" method (Fig. 3). Its essence is as follows. Suppose that of three amino acids (A, B and C) you need to get all possible tripeptide combinations. For this, the granules of the solid polymer carrier (P) are separated by three equal portions and are treated with a solution of one of the amino acids. In this case, all amino acids are chemically associated with the surface of the polymer one of its functional groups. The obtained polymers of three varieties are thoroughly mixed, and the mixture is again separated into three parts. Then each part containing all three amino acids in the same quantities is again treated with one of the same three amino acids and receive nine dipeptides (three mixtures of three products). Another mixture, the separation into three equal parts and the treatment of amino acids give the desired 27 tripipeptides (three mixtures of nine products) in just nine stages, while the receipt of them would require the synthesis of 27 × 3 \u003d 81 stages.
Ministry of Education and Science of the Russian Federation
FGAOU VPO "Ural Federal University named after the first president of Russia B. N. Yeltsin"
Department of Organic Synthesis Technology
Abstract on the topic: "Principles and methods of solid phase synthesis. Synthesis peptides "
Performed student c. X-300803.
Shaikutdinova A.I.
Checked Berseneva V.S.
Yekaterinburg 2013.
1. Introduction .................................................................................... 3
2. What is peptides? .............................................. ..............................................four
2.1. The structure of peptides ................................................................5
2.2. Synthesis of peptides .................................................................. .7
3. solid-phase synthesis of peptides ................................................... 10
3.1. Method of Merrinfield ............................................................ 10
3.2. Solid substrate ................................................................14
3.3. Choosing a substrate ............................................................... ... 14
3.4. Linkers ............................................................................16
4. The first synthesis of natural hormone - oxytocin .............................22
5. Synthesis insulin in a cage ....................................................................... ..30
6. Conclusion ....................................................................................... ..34
7. Literature ........................................................................... ... 35
Introduction
In organic chemistry there is not a single reaction that provides in practice quantitative yield of target products anyway. The only exception is apparently the total combustion of organic substances in oxygen at high temperatures up to CO 2 and H 2 O. Therefore, cleaning the target product is a complex and time-consuming task. For example, 100% purification of peptide synthesis products is a difficult problem. Indeed, the first full synthesis of peptide, an oxytocin hormone (1953 g) containing only 8 amino acid residues, was considered as an outstanding achievement that brought him to the author, V. Du Vino, the Nobel Prize of 1955. However, in the next twenty years, the synthesis of polypeptides of such complexity turned In the routine, so now the synthesis of polypeptides consisting of 100 and more amino acid residues is no longer considered as an irresistible difficult task.
Objective: Disassemble and explain: "What caused such dramatic changes in the field of polypeptide synthesis?"
What is peptides?
Peptides - natural or synthetic compounds,moleculeswhich are built from residuesalpha-amino acids interconnected by peptide (amide) bonds C (O) NH. May contain B.moleculealso an uninuted component (eg, the residuecarbohydrate). By the number of amino acid residues included inmoleculespeptides distinguish dipeptides, tripipeptides, tetrapeptides, etc. Peptides containing up to 10 amino acid residues are called oligopeptides containing more than 10 amino acid residues of natural polypeptides polypeptides.with a molecular weight of more than 6 thousand calledproteins.
For the first time, peptides were isolated from enzymatic protein hydrolyzes. The term "peptides" is proposed by E. Fisher. The first synthetic peptide received T. Kursius in 1881. E. Fisher by 1905 developed the first general method of synthesis of peptides and synthesized a number of oligopeptides of various buildings. The existing contribution to the development of chemistry of peptides made students E. Fisher E. Abdergalden, Lake and M. Bergman. In 1932, M Bergman and L. Zervas was used in the synthesis of peptides benzyloxycarbonyl group (carbobenzoxy group) to protect the alpha-amino groups of amino acids, which marked a new stage in the development of peptide synthesis. The obtained N-protected amino acids (N-carbobenzoxyamin acids) were widely used to obtain different peptides, which were successfully used to study a number of key problems of chemistry and biochemistry of these substances, for example, to study the substrate specificity of proteolytic enzymes. With the use of N-carboenzoxyamin acids, natural peptides (glutathione, carnosine, etc.) were first synthesized. An important achievement in this area developed in the early 50s. P. Vogan and others. Peptide synthesis by mixed anhydrides.
In 1953 V. Du Vino synthesized the first peptide hormone-toxotcin. Based on the concepts developed by P. Merryphield in 1963, automatic peptide synthesizers were created. Received intensive development methods of controlled enzymatic synthesis of peptides. The use of new methods made it possible to carry out the synthesis of the hormone insulin, etc.
The successes of synthetic chemistry of peptides were prepared by achievements in the development of such methods of separation, purification and analysis of peptides, such as ion exchange chromatography, electrophoresis on various carriers, gel filtration, highly efficient liquid chromatography (HPLC), immuno-chemical analysis, etc. Received great development Methods for analyzing the end groups and methods of stepped peptide splitting. There were, in particular, automatic amino acid analyzers and automatic instruments were created to determine the primary structure of peptide-so-called sequectors.