Types of covalent bonds polar non-polar HCl, H2O H2, CL2, N2. Chemical Communication HCl ion connection
Task number 1
From the proposed list, select two compounds in which ionic chemical bond is present.
- 1. CA (CLO 2) 2
- 2. HCLO 3.
- 3. NH 4 Cl
- 4. HCLO 4.
- 5. Cl 2 O 7
Answer: 13.
It is possible to determine the presence of an ionic type of communication in compound in the overwhelming majority of cases, it is possible for the composition of its structural units at the same time the atoms of typical metal and the non-metal atoms are included.
On this basis, we establish that ionic communication is available in a compound at number 1 - Ca (CLO 2) 2, because In its formula, you can see atoms of a typical calcium metal and non-metallulov atoms - oxygen and chlorine.
However, more compounds containing at the same time atoms of metal and nonmetalla, in the specified list item.
Among the compounds indicated in the task there is ammonium chloride, in it an ionic connection is implemented between the ammonium cation of NH 4 + and the CL chloride-ion.
Task number 2.
From the proposed list, select two compounds in which the type of chemical bond is the same as in the fluorine molecule.
1) Oxygen
2) nitrogen oxide (II)
3) Bromomopod
4) Sodium iodide
Record the number of selected connections in the response field.
Answer: 15.
Fluorine (F 2) molecule consists of two atoms of one chemical element of non-metal, so chemical bond in this molecule is covalent, non-polar.
Covenate non-polar communication can be implemented only between atoms of the same chemical element of non-metal.
Of the proposed options, the covalent unpolar type of communication has only oxygen and diamond. The oxygen molecule is a dioxide, consists of atoms of a single chemical element of non-metal. Diamond has an atomic structure and in its structure every carbon atom, which is non-metal, is associated with 4 other carbon atoms.
Nitrogen oxide (II) is a substance consisting of molecules formed by atoms of two different non-metals. Since electronegability of different atoms are always different, the overall electron pair in the molecule is shifted to a more electronegative element, in this case to oxygen. Thus, the connection in the NO molecule is a covalent polar.
Bromomopod also consists of diatomic molecules consisting of hydrogen and bromine atoms. The total electron pair forming the H-BR connection is shifted to a more electronegative bromine atom. Chemical bond in the HBr molecule is also a covalent polar.
Sodium iodide is an ionic structure formed by a metal cation and anion ion. Communication in the NAI molecule is formed by the transition of an electron from 3 s.-The sodium atoms (sodium atom turns into a cation) on a fallen 5 p.-Orbital atom of the iodine (the atom of the iodine turns into an anion). Such a chemical connection is called ion.
Task number 3.
From the proposed list, select two substances between the molecules of which hydrogen bonds are formed.
- 1. C 2 H 6
- 2. C 2 H 5 OH
- 3. H 2 O
- 4. CH 3 OCH 3
- 5. CH 3 COCH 3
Record the number of selected connections in the response field.
Answer: 23.
Explanation:
Hydrogen bonds take place in the substances of the molecular structure, in which the h-o, h - n, h-f covalet bonds are present. Those. Covalent bonds of hydrogen atom with atoms of three chemical elements with the highest electronegathy.
Thus, obviously, hydrogen bonds are between molecules:
2) Alcohol
3) Fenolov
4) carboxylic acids
5) Ammonia
6) primary and secondary amines
7) Plastic Acid
Task number 4.
From the proposed list, select two compounds with ion chemical bond.
- 1. PCL 3.
- 2. CO 2.
- 3. NaCl
- 4. H 2 S
- 5. MGO.
Record the number of selected connections in the response field.
Answer: 35.
Explanation:
It is possible to conclude about the presence of an ionic type of communication in the compound in the overwhelming majority of cases, it is possible for the composition of the structural units of the substance at the same time the atoms of typical metal and non-metal atoms are included.
On this basis, we establish that ion connection is available in a connection at number 3 (NaCl) and 5 (MGO).
Note*
In addition to the above feature, the presence of ion bonds in the compound can be said if the composition of its structural unit contains ammonium cation (NH 4 +) or its organic analogs - alkylammonium cations RNH 3 +, dialkylamonia R 2 NH 2 +, trialkilammonium R 3 NH + or tetraalklammonium R 4 n +, where R is some hydrocarbon radical. For example, the ion type of communication takes place in compound (CH 3) 4 NCL between the cation (CH 3) 4 + and the CL chloride-ion.
Task number 5.
From the proposed list, select two substances with the same type of structure.
4) Salt Salt
Record the number of selected connections in the response field.
Answer: 23.
Task number 8.
From the proposed list, select two substances of the non-elastic structure.
2) Oxygen
3) White Phosphorus
5) silicon
Record the number of selected connections in the response field.
Answer: 45.
Task number 11.
From the proposed list, select two substances in the molecules of which there is a double bond between carbon and oxygen atoms.
3) formaldehyde
4) acetic acid
5) Glycerin
Record the number of selected connections in the response field.
Answer: 34.
Task number 14.
From the proposed list, select two substances with ion bond.
1) Oxygen
3) carbon oxide (IV)
4) sodium chloride
5) calcium oxide
Record the number of selected connections in the response field.
Answer: 45.
Task number 15.
From the proposed list, select two substances with the same type of crystal lattice as the diamond.
1) SiO 2 silica
2) Sodium oxide Na 2 O
3) Curmarket gas CO
4) White phosphorus P 4
5) silicon Si
Record the number of selected connections in the response field.
Answer: 15.
Task number 20.
From the proposed list, select two substances in which there are one triple molecules.
- 1. HCOOH
- 2. HCOH
- 3. C 2 H 4
- 4. N 2.
- 5. C 2 H 2
Record the number of selected connections in the response field.
Answer: 45.
Explanation:
In order to find the correct answer, draw the structural formulas of the connections from the list presented:
Thus, we see that the triple bond is available in nitrogen and acetylene molecules. Those. Right answers 45.
Task number 27.
From the proposed list, select two substances in the molecules of which there is a covalent non-polar connection.
1.Telectric earth metals are related5) to s- elements
6) to p- elements
7) to D- elements
8) to f - elements
2. How many electrons contain atoms of alkaline earth metals in the external energy level
1) one 2) two 3) three 4) four
3. In chemical reactions, aluminum atoms show
3) Oxidative Properties 2) Acid Properties
4) 3) Recovery Properties 4) Basic Properties
4. Calcium interaction with chlorine refers to reactions
1) decomposition 2) compound 3) substitution 4) exchange
5. The molecular weight of sodium bicarbonate is equal to:
1) 84 2) 87 3) 85 4) 86
3. What atom is heavier - iron or silicon - and how many times?4. Consider relative molecular weights of simple substances: hydrogen, oxygen, chlorine, copper, diamond (carbon). Recall which of them consist of diatomic molecules, and which are from atoms.
5. Distribute relative molecular weights of the following compounds of CO2 carbon dioxide sulfuric acid H2SO4 Sugar C12H22O11 Ethyl alcohol C2H2O marble sacro3
6. In a hydrogen peroxide, one oxygen atom accounts for one hydrogen atom. Determine the formula of hydrogen premission, if it is obliged that its relative molecular weight is 34. What is the mass ratio of hydrogen and oxygen in this connection?
7. How many times is the carbon dioxide molecule heavier oxygen molecule?
Help the lifeguard, task 8 class.
Chemical bond.
Exercises.
1. Determine the type of chemical bond in the following substances:
Substance | Chloride phosphorus | Sulfuric acid | |||
Type of Communication | |||||
Substance | Barium oxide | ||||
Type of Communication |
2. Stress substances in which Between molecules exists hydrogen communications:
sulphur dioxide; ice; ozone; ethanol; ethylene; acetic acid; fluoropod.
3. How affects length, strength and polarity of communication - Radius of atoms, their electronegability, multiplicity of communication?
but) The more radii atoms who formed communication length communication _______
b) The greater the multiplicity (single, double or triple) connection, the strength ____________________
in) The greater the difference of electronegate Between two atoms, the polarity of communication ____________
4. Compare Length, strength and polarity of connections in molecules:
a) Communication Length: HCl ___HBR
b) communication strength p3_______nh3
c) Polarity of CCL4 Communication ______CH4
d) Communication strength: N2 _______O2
e) the length of the communication between carbon atoms in ethylene and in acetylene: __________
e) polarity of connections in NH3 _________ Н2O
Tests. A4.Chemical connection.
1. The valence of the atom is
1) the number of chemical bonds formed by this atom in the compound
2) the degree of oxidation of the atom
3) the number of given or received electrons
4) the number of electrons missing to obtain an electronic configuration of the nearest inert gas
A. In the formation of chemical bond, energy always stands out
B. The dual bond energy is less than the energy of single communication.
1) It is true only a 2) is true only b 3) both judgments 4) both judgments are incorrect
3.In the substances formed by connecting same atoms, chemical
1) ionic 2) covalent polar 3) hydrogen 4) covalent non-polar
4. Compounds with covalent polar and covalent non-polar bonds are respectively
1) water and hydrogen sulfide 2) bromide potassium and nitrogen
5. Due to the general electronic pair, a chemical connection is formed in conjunction
1) ki 2) HBr 3) Li2O 4) Navr
6. Select a couple of substances, all links in which are covalent:
1) NASL, NSL 2) CO2, WA 3) CH3SL, CH3NA 4) SO2, NO2
7. An exercise with a covalent polar communication has a formula
1) KCl 2) HBr 3) P4 4) CaCl2
8. Connection with the ionic character of the chemical
1) phosphorus chloride 2) Bromide potassium 3) nitrogen oxide (II) 4) barium
9. In ammonia and chloride Barium Chemical Communication, respectively
1) ionic and covalent polar 2) covalent non-polar and ionic 3) covalent polar and ionic 4) covalent non-polar and metal
10. A substance with a covalent polar bond is
1) sulfur oxide (IV) 2) oxygen 3) calcium hydride 4) diamond
11. In which row lists the substances only with a covalent polar bond:
1) CH4 H2 SL2 2) NH3 HBr CO2 3) PCL3 KCL CCL4 4) H2S SO2 LIF
12. In which row lists the substances only with the ion type of communication:
1) F2O LIF SF4 2) PCL3 NaCl CO2 3) KF Li2O BACL2 4) Saf2 CH4 CCL4
13. Connection with ion connection is formed when interaction
1) CH4 and O2 2) NH3 and HCl 3) C2H6 and HNO3 4) SO3 and H2O
14. In which substance, all chemical connections are covalent unpolar?
1) Diamond 2) Carbon Oxide (IV) 3) Gold 4) Methane
15. Communication formed between elements with sequence numbers 15 and 53
1) ionic 2) metal
3) Covenate non-polar 4) covalent polar
16. Hydrogen communications Forms between Molecules
1) ethane 2) benzene 3) hydrogen 4) ethanol
17. In which substance is hydrogen bonds?
1) hydrogen sulfide 2) ice 3) bromomopod 4) benzene
18. In what substance are at the same time ion and covalent chemical connections?
1) sodium chloride 2) chloride hydrogen sodium sulfate 4) phosphoric acid
19. A more pronounced ionic character has a chemical connection in the molecule
1) Lithium Bromide 2) Copper Halrid 3) Calcium Carbide 4) Potassium Fluoride
20. Three common electronic pairs formed a covalent bond in a molecule 1) of nitrogen 2) hydrogen sulfide 3) methane 4) chlorine
21. How much does the electrons participate in the formation of chemical bonds in the water molecule? 4) 18
22. Type Covalent bonds contains molecule: 1) CO2 2) C2H4 3) P4 4) C3N4
23. The number of connections in molecules increases in a number
1) SNSL3, CH4 2) CH4, SO3 3) CO2, CH4 4) SO2, NN3
24. In what compound the covalent bond between atoms is formed according to the donor-acceptor mechanism? 1) KSL 2) CCL4 3) NN4SL 4) SASL2
25. Which of the listed molecules requires the smallest energy costs for decomposition on atoms? 1) Hi 2) H2 3) O2 4) with
26. Specify a molecule in which communication energy is the greatest:
1) N≡n 2) N-H 3) O \u003d O 4) H-F
27. Specify the molecule in which the chemical connection is the most durable:
1) HF 2) NSL 3) HBR 4) Hi
28. Specify a series characterized by an increase in chemical communication
1) O2, N2, F2, CL2 2) N2, O2, F2, CL2 3) F2, N2, O2, CL2 4) N2, O2, CL2, F2
29. E-O communication length increases in row
1) silicon oxide (IV), carbon oxide (IV)
2) Sulfur Oxide (IV), Tellur Oxide (IV)
3) strontium oxide, beryllium oxide
4) sulfur oxide (IV), carbon oxide (IV)
30. In a series of CH4 - Sih4 occurs increase
1) tensile strength 2) oxidative properties
3) Length of bonds 4) polarity of ties
31. In which row of molecules are located in order to increase the polarity of connections?
1) HF, NSL, HBR 2) H2SE, H2S, H2O 3) NH3, pH3, ASN3 4) CO2, CS2, CSE2
32. The most polar covalent bond in the molecule:
1) CH4 2) CF4 3) CCL4 4) CBR4
33. Here a number in which polarity increases:
1) AGF, F2, HF 2) CL2, HCl, NaCl 3) CUO, CO, O2 4) KBR, NaCl, KF
Covalent chemical bond, its varieties and education mechanisms. Characteristics of covalent bond (polarity and communication energy). Ion connection. Metal connection. Hydrogen bond.
1. In ammonia and chloride Barium Chemical Communication, respectively
1) ionic and covalent polar
2) covalent polar and ionic
3) Covenant non-polar and metal
4) Covenant non-polar and ionic
2. Substances only with ion bond are given in a series:
1) F2, CL4, ks1
2) NaBr, Na2O, Ki
3. The connection with the ion bond is formed when interacting
3) C2H6 and HNO3
4. In which row all substances have a covalent polar communication?
1) HCl, NaCl. CL2.
4) NaBr. HBr. Co.
5. In which formulas of substances are recorded only with covalent polar
1) C12, NO2, NS1
6. Covalent non-polar connection is characteristic of
1) C12 2) SO3 3) CO 4) SiO2
7. Substance with a covalent polar communication is
1) C12 2) NABR 3) H2S 4) MgCl2
8. The covalent bond substance is
1) SAS12 2) MGS 3) H2S 4) NABR
9. The substance with a covalent non-polar connection has a formula
1) NH3 2) Cu 3) H2S 4) i2
10. Substances with non-polar covalent bond are
1) water and diamond
2) hydrogen and chlorine
3) copper and nitrogen
4) bromine and methane
11. Chemical Communication is formed between atoms with the same relative electronegility.
2) covalent polar
3) Covalent Nonolaur
4) hydrogen
12. Covalent polar bond is characteristic of
1) kc1 2) nvg 3) Р4 4) sasl2
13. The chemical element in the atom of which electrons by layers are distributed as follows: 2, 8, 8, 2 forms chemical communication with hydrogen
1) covalent polar
2) Covalent Nonolaur
4) Metallic
14. In which substance molecule, the length of the connection between carbon atoms is the highest?
1) acetylene 2) ethane 3) ethen 4) benzene
15. Three common electronic pairs formed a covalent bond in the molecule
2) Serovodorod.
16. Hydrogen bonds are formed between molecules
1) dimethyl ether
2) methanol
3) ethylene
4) ethyl acetate
17. Polarity of communication is most pronounced in the molecule
1) Hi 2) ns1 3) hf 4) nvg
18. Substances with non-polar covalent bond are
1) water and diamond
2) hydrogen and chlorine
3) copper and nitrogen
4) bromine and methane
19. Hydrogen bond is not typical for substance
1) H2O 2) CH4 3) NH3 4) Snzon
20. Covalent polar bond is characteristic of each of the two substances whose formulas
2) CO2 and K2O
4) CS2 and PC15
21. Least durable chemical connection in the molecule
1) fluorine 2) chlorine 3) bromine 4) iodine
22. In what substance molecule is the highest chemical connection length?
1) fluorine 2) chlorine 3) bromine 4) iodine
23. Covalent bonds has each of the substances specified in the row:
1) C4H10, NO2, NaCl
2) CO, CUO, CH3CL
4) C6H5NO2, F2, CC14
24. Covalent bond has each of the substances specified in the row:
1) Sao, C3N6, S8
2) FE. Nano3, Co.
3) N2, Cuco3, K2S
4) C6H5N02, SO2, CHC13
25. Covalent bond has each of the substances specified in the row:
1) C3N4, NO, NA2O
2) CO, CH3S1, PBR3
3) R2oz, NaHSO4, Cu
4) C6H5NO2, NAF, CC14
26. Covalent bonds has each of the substances specified in the Row:
1) C3HA, NO2, NAF
2) KS1, CH3CL, C6H12O6
3) P2O5, NaHSO4, BA
4) C2H5NH2, P4, CH3OH
27. Polarity of communication is most pronounced in molecules
1) Serovodorod.
3) Phosphine
4) Hloreodor
28. In what substance molecule, the chemical bonds are the most durable?
29. Among the substances NH4Cl, CSCl, NanO3, PH3, HNO3 - the number of compounds with ion connection is equal
30. Among substances (NH4) 2SO4, Na2SO4, CAI2, I2, CO2 are the number of connections with a covalent bond equal to
Answers: 1-2, 2-2, 3-4, 4-3, 5-4, 6-1, 7-3, 8-3, 9-4, 10-2, 11-3, 12-2, 13-3, 14-2, 15-1, 16-2, 17-3, 18-2, 19-2, 20-4, 21-4, 22-4, 23-4, 24-4, 25- 2, 26-4, 27-4, 28-1, 29-3, 30-4
The unified theory of chemical bond does not exist, conditionally chemical bonds are divided into a covalent (universal type of communication), ionic (private case of covalent bond), metallic and hydrogen.
Covalent communication
The formation of covalent communication is possible in three mechanisms: exchange, donor-acceptor and dative (Lewis).
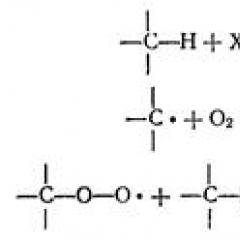
According to exchange mechanism The formation of covalent communications occurs due to the generalization of common electronic pairs. At the same time, each atom seeks to purchase an inert gas shell, i.e. Get a completed external energy level. The formation of chemical bonds on exchange type is depicted using Lewis formulas, in which each valence electro atom is depicted by points (Fig. 1).
Fig. 1 Education of a covalent bond in the HCl molecule on the exchange mechanism
With the development of the theory of the structure of the atom and quantum mechanics, the formation of a covalent bond is represented as overlapping electronic orbitals (Fig. 2).

Fig. 2. Education of covalent communication due to overlapping electronic clouds
The greater the overlapping of atomic orbitals, the stronger the connection, less the length of the communication and its more energy. Covalent bond can be formed by overlapping different orbital. As a result of the overlap of S-S, S-P orbitals, as well as D-D, P-P, D-P orbitals by side blades, education occurs. Perpendicular to the line connecting the core of 2 atoms is formed - the connection. One - and one - the relationship is capable of forming a multiple (double) covalent bond, characteristic of the organic substances of the class of alkenes, alkadiennes, etc. One - and two - connections form a multiple (triple) covalent bond, characteristic of the organic substances of the alkine class (acetylenes).
Education covalent donor-acceptor mechanism Consider on the example of ammonium cation:
NH 3 + H + \u003d NH 4 +
7 N 1S 2 2S 2 2P 3

The nitrogen atom has a free marginal pair of electrons (electrons not participating in the formation of chemical bonds inside the molecule), and hydrogen cation is a free orbital, so they are a donor and an electron acceptor, respectively.
The duty mechanism for the formation of a covalent connection will consider on the example of the chlorine molecule.
17 Cl 1S 2 2S 2 2P 6 3S 2 3P 5

The chlorine atom has a free marginal pair of electrons and vacant orbital, therefore, can show properties and donor and acceptor. Therefore, in the formation of a chlorine molecule, one chlorine atom acts as a donor, and the other is an acceptor.
Main characteristics of covalent bond are: saturability (rich connections are formed when the atom attaches to itself so much electrons as its valence capabilities allow; unsaturated bonds are formed when the number of electrons connected is less than the flu valence capabilities); Direction (this value is associated with the geometry of the molecule and the concept of "valence angle" - angle between connections).
Ion communication
There are no compounds with a pure ion bond, although this is a chemically related state of atoms, in which the steady electron environment of the atom is created with a full transition of a general electron density to an atom of a more electronegative element. Ionic communication is possible only between the atoms of electronegative and electropositive elements that are in a state of variemetically charged ions - cations and anions.
Definition
Ion Called electrically charged particles formed by separating or attaching an electron to the atom.
When the electron is transmitted, the atoms of metals and non-metals tend to form a stable configuration of the electronic shell around their kernel. The nenetal atom creates a shell of the subsequent inert gas around its kernel, and the metal atom is the previous inert gas (Fig. 3).

Fig. 3. Education of ion communication on the example of sodium chloride molecule
Molecules in which in pure form there is an ion connection are found in a vapor state of the substance. The ionic relationship is very durable, in connection with this substance with this bond have a high melting point. In contrast to covalent for ionic communication, direction and saturation is not characteristic, since the electric field created by ions acts the same to all ions due to spherical symmetry.
Metal connection
Metal bond is realized only in metals - this is the interaction that holds the metals atoms in a single lattice. Only valence electrons of metal atoms belonging to all its volume are involved in the formation of communication. In metals from atoms, electrons are constantly separated, which move throughout the entire mass of the metal. Metal atoms, devoid of electrons, are converted into positively charged ions that seek to accept moving electrons. This continuous process forms inside the metal so-called "electronic gas", which firmly connects all atoms of metal (Fig. 4).
The metal bond is strong, therefore, the metals are characterized by a high melting point, and the presence of "electronic gas" gives the metals with pupidity and plasticity.
Hydrogen communications
Hydrogen bond is a specific intermolecular interaction, because Its occurrence and strength depend on the chemical nature of the substance. It is formed between molecules in which a hydrogen atom is associated with an atom with high electronegitability (O, N, S). The occurrence of the hydrogen bond depends on two reasons, firstly, the hydrogen atom associated with an electronegative atom does not have electrons and can easily be embedded in electronic clouds of other atoms, and, secondly, possessing a valence S-orbital, a hydrogen atom can take a watery pair electrons of the electronegative atom and to form a bond with a donorly acceptor mechanism with it.
169338 0
Each atom has some number of electrons.
When entering into chemical reactions, the atoms are given, they acquire, or communicate electrons, reaching the most stable electronic configuration. The most stable is the configuration with the lowest energy (as in the atoms of noble gases). This pattern is called "octet rules" (Fig. 1).
Fig. one.
This rule applies to all types of connections. Electronic connections between atoms allow them to form stable structures, from the simplest crystals to complex biomolecules forming, ultimately live systems. They differ from crystals with continuous metabolism. In this case, many chemical reactions proceed by mechanisms electronic transferwho play a crucial role in the energy processes in the body.
Chemical bond is a force that holds two or more atoms, ions, molecules or any combination.
The nature of the chemical bond is universal: this is an electrostatic force of attraction between negatively charged electrons and positively charged nuclei, determined by the configuration of electrons of the outer shell of atoms. The ability of an atom to form chemical connections is called valence, or degree of oxidation. With valence associated with the concept of valence electrons - electrons forming chemical bonds, that is, located at the most high-energy orbital. Accordingly, the outer shell of the atom containing these orbital is called valentine's sheath. Currently, it is not enough to indicate the presence of a chemical bond, and it is necessary to clarify its type: ionic, covalent, dipole-dipole, metallic.
First type of communication -ionic communication
In accordance with the electronic theory of Lewis and Kossel's valence, atoms can achieve a stable electronic configuration in two ways: first, losing electrons, turning into cations, secondly, acquiring them, turning into anions. As a result of electronic transfer, thanks to the electrostatic strength of attraction between ions with charges of the opposite sign, a chemical bond called the cossel " electrovalent"(Now it is called ionic).
In this case, the anions and cations form a stable electronic configuration with an external electronic shell filled. Typical ionic bonds are formed from cations T and II groups of periodic system and anions of non-metallic elements VI and VII groups (16 and 17 subgroups - respectively, chalcogenovand halogen). Communication in ionic compounds are unsaturated and non-directional, so the possibility of electrostatic interaction with other ions is preserved. In fig. 2 and 3 are examples of ionic connections that correspond to the electronic co-axle transfer models.
Fig. 2.
Fig. 3. Ion connection in the table salt molecule (NaCl)
Here it is appropriate to remind some properties that explain the behavior of substances in nature, in particular, consider the idea of acidsand basins.
The aqueous solutions of all these substances are electrolytes. They change in different ways indicators. The mechanism of action of indicators was opened by F.V. Ostelad. It showed that the indicators are weak acids or bases, the painting of which is dissolved in the unfair and dissociated states.
The bases are able to neutralize the acids. Not all bases are soluble in water (for example, non-soluble, some organic compounds that do not contain - on-groups, in particular, triethylamine N (C 2N 5) 3); Soluble bases are called alkalis.
Aqueous solutions acids enter the characteristic reactions:
a) with metal oxides - with the formation of salt and water;
b) with metals - with the formation of salt and hydrogen;
c) with carbonates - with salt formation, Co. 2 I. N. 2 O..
The properties of acids and bases describe several theories. In accordance with the theory of S.A. Arrhenius, acid is a substance that dissociates with the formation of ions N. +, whereas the base forms ions IS HE -. This theory does not take into account the existence of organic bases that do not have hydroxyl groups.
In accordance with S. protonnathe theory of Brensted and Lowry, acid is a substance containing molecules or ions that give protons ( donorsprotons), and the base is a substance consisting of molecules or ions taking protons ( acceptorsprotons). Note that in aqueous solutions of hydrogen ions exist in hydrated form, that is, in the form of hydroxony ions H 3 O. +. This theory describes the reaction not only with water and hydroxide ions, but also carried out in the absence of a solvent or with a non-aqueous solvent.
For example, in the reaction between ammonia NH 3 (weak base) and the chloride in the gas phase is formed solid ammonium chloride, and 4 particles are always present in an equilibrium mixture of two substances, two of which are acids, and the other - bases:
This equilibrium mixture consists of two conjugate pairs of acids and bases:
1) NH 4 + I. NH 3
2) HCLand Cl ‑
Here in each conjugate pair of acid and the base differ on one proton. Each acid has a conjugate base. A weak conjugate base corresponds to severe acid, and a severe conjugate base.
The theory of Brensteda Lowei allows you to explain the uniqueness of the role of water for the livelihood of the biosphere. Water, depending on the substance interacting with it, can exhibit properties or acids, or base. For example, in reactions with aqueous solutions of acetic acid, water is the base, and with an aqueous solutions of ammonia - acid.
1) CH 3 coxy + H 2 O. ↔ H 3 O. + + CH 3 SOO -. Here, the molecule of acetic acid is by the proton of the water molecule;
2) NH 3. + H 2 O. ↔ NH 4. + + IS HE -. Here, the ammonia molecule accepts the proton from the water molecule.
Thus, water can form two conjugate pairs:
1) H 2 O. (acid) and IS HE - (conjugate base)
2) H 3 O. + (acid) and H 2 O.(conjugate base).
In the first case, the water is diagnosed with proton, and in the second - accepts it.
This property is called amphiprotonality. Substances that can enter into reactions in quality and acids and grounds are called amphoteric. In the wilderness, such substances are common. For example, amino acids are capable of forming salts and with acids, and with bases. Therefore, peptides easily form coordination compounds with those present metal ions.
Thus, the characteristic property of the ion connection is the complete movement of the naps of the binding electrons to one of the cores. This means that there is an area between ions, where the electronic density is almost zero.
Second Communication Type -covalent communication
Atoms can form stable electronic configuration by combining electrons.
Such a connection is formed when the pair of electrons is generalized by one from everyone Atom. In this case, the Common Communication Electrons are distributed between atoms equally. Examples of covalent communication can be called gomoidernydihomatomy molecules N. 2 , N. 2 , F. 2. The same type of communication is available at allotropics O. 2 and ozone O. 3 and in the polyatomic molecule S. 8, as well as heteroantore molecules chloroodor Nsl, carbon dioxide Co. 2, metha SH 4, ethanol FROM 2 N. 5 IS HE, sulfur hexafluoride Sf. 6, acetylene FROM 2 N. 2. In all these molecules, electrons are equally common, and their connections are saturated and directed equally (Fig. 4).
For biologists, it is important that in double and triple bonds, covalent radii atoms are reduced compared to single bond.
Fig. four. Covalent bond in the CL 2 molecule.
The ionic and covalent types of connections are two limiting cases of many existing types of chemical bonds, and in practice most intermediate bonds.
The compounds of two elements located in the opposite ends of one or different periods of the Mendeleev system are preferably forming ionic ties. As it rates the elements within the period, the ionic nature of their compounds is reduced, and covalent - increases. For example, halides and oxides of elements of the left part of the periodic table form predominantly ionic connections ( NaCl, Agbr, Baso 4, Caco 3, Kno 3, Cao, Naoh), and the same connections of the elements of the right part of the table - covalent ( H 2 O, CO 2, NH 3, NO 2, CH 4, phenol C 6 H 5 OH, glucose C 6 H 12 O 6, ethanol From 2N 5 he).
A covalent bond, in turn, has another modification.
In polyhytomic ions and in complex biological molecules, both electrons can occur only from oneatom. It is called donorelectronic couple. Atom, a compatible with a donor of this pair of electrons, is called acceptorelectronic couple. Such a kind of covalent communication is named coordination (donor-acceptor, ordative) commonwealth(Fig. 5). This type of communication is most important for biology and medicine, since the chemistry of the most important D-elements for metabolism is largely described by coordination bonds.
PC. five.
As a rule, in the complex compound, the metal atom acts as an acceptor of an electronic pair; On the contrary, with ionic and covalent bonds, the metal atom is an electron donor.
The essence of the covalent bond and its varieties - coordination communications - can be clarified with the help of another theory of acids and the grounds proposed by GG. Lewis. He somewhat expanded the semantic concept of the terms "Acid" and "Base" on the theory of Brenstead-Lowry. Lewis's theory explains the nature of the formation of complex ions and the participation of substances in the reactions of nucleophilic substitution, that is, in the formation of the COP.
According to Lewis, acid is a substance capable of forming a covalent connection by accepting an electronic pair from the base. The Lewis base is called a substance with a mean-free electron pair, which, by turning the electrons, forms a covalent bond with Lewisic acid.
That is, Lewis theory expands the circle of acid-base reactions also on the reaction in which protons do not participate at all. Moreover, the proton itself, according to this theory, is also acid, since it is capable of accepting an electronic pair.
Consequently, according to this theory, cations are leewasic acids, and anions are Lewis bases. An example is the following reactions:
It is noted above that the subdivision of substances to ionic and covalent relative, since the complete transition of the electron on the metal atoms to acceptor atoms in covalent molecules does not occur. In compounds with ion bond, each ion is located in the electric field of the ions of the opposite sign, so they are mutually polarized, and their shells are deformed.
Polarizabilitydetermined by the electronic structure, charge and sizes of the ion; Anions are higher than that of the cations. The greatest polarizability among cations - the cations of a larger charge and smaller, for example, HG 2+, CD 2+, PB 2+, Al 3+, TL 3+. A strong polarizing action possesses N. +. Since the influence of the polarization of the ions is bilateral, it significantly changes the properties of the compounds formed by them.
Third Communication Type -dipole-dipole communication
In addition to the listed types of communication, the dipole-dipole distinguish intermolecularinteractions called also vantherval masses .
The strength of these interactions depends on the nature of molecules.
Mix the interactions of three types: Permanent dipole - Permanent dipole ( dipole-dipole attraction); Permanent dipole - induced dipole ( induction attraction); Instant dipole - induced dipole ( dispersion attraction, or London forces; Fig. 6).
Fig. 6.
Dipole-dipole moment possess only molecules with polar covalent bonds ( HCl, NH 3, SO 2, H 2 O, C 6 H 5 Cl), and the communication force is 1-2 debay(1D \u003d 3.338 × 10 -30 pendant meter - CL × M).
In biochemistry, one more type of communication is distinguished - hydrogen communication, which is an extreme case dipole-dipole attraction. This relationship is formed by attraction between the hydrogen atom and the electronegative atom of a small size, most often - oxygen, fluorine and nitrogen. With large atoms with similar electronegility (for example, with chlorine and gray), hydrogen bond is significantly weaker. The hydrogen atom is characterized by one essential feature: when it is distinguished by the binding electrons, its kernel - proton - is taken off and ceases to be applied by electrons.
Therefore, the atom turns into a major dipole.
Hydrogen bond, unlike Vanderwals, is formed not only for intermolecular interactions, but also inside one molecule - intramolecularhydrogen bond. Hydrogen bonds play an important role in a biochemistry, for example, to stabilize the structure of proteins in the form of a-helix, or for the formation of a double DNA helix (Fig. 7).
Fig.7.
Hydrogen and Vanderwalts bonds are much weaker than ionic, covalent and coordination. The energy of intermolecular connections is indicated in Table. one.
Table 1. Energy of intermolecular power
Note: The degree of intermolecular interactions reflect the indicators of the enthalpy of melting and evaporation (boiling). Ion compounds are required to separate ions much more energy than for the separation of molecules. Enthalpy melting ionic compounds is significantly higher than molecular compounds.
Fourth Communication Type -metal communication
Finally, there is another type of intermolecular ties - metal: Communication of positive metal lattice ions with free electrons. In biological objects, this type of communication is not found.
From a brief overview of types of bonds, one piece is found: an important parameter of an atom or metal ion - electrons donor, as well as an atom - electron acceptor is its the size.
Without going into details, we note that the covalent radii of atoms, the ionic radii of metals and Vanderwali radii of interacting molecules increase as they increase their sequence number in the periodic groups. At the same time, the values \u200b\u200bof the radii ions are the smallest, and the vantherwalvas radius - the largest. As a rule, when moving down the group, the radii of all elements increase, both both covalent and Vanderwals.
The greatest value for biologists and doctors have coordination(donor-acceptor) Communications considered by coordination chemistry.
Medical bioornery. GK Barashkov