Reactions replacement of ligands. Reactivity complexes
General Chemistry: Textbook / A. V. Zhulkhan; Ed. V. A. Popkov, A.V. Zhoglovna. - 2012. - 400 p.: Il.
Chapter 7. Complex Compounds
Chapter 7. Complex Compounds
Comprehensive elements are organizers of life.
K. B. Yatimirsky
Complex compounds are the most extensive and diverse class of compounds. In living organisms, complex compounds of biogenic metals with proteins, amino acids, porphy-rins, nucleic acids, carbohydrates, macrocyclic compounds are present. The most important processes of vital activity proceed with the participation of complex compounds. Some of them (hemoglobin, chlorophyll, hemocyanin, vitamin B 12, etc.) play a significant role in biochemical processes. Many drugs contain metals complexes. For example, insulin (zinc complex), vitamin B 12 (Cobalt complex), Platinol (platinum complex), etc.
7.1. Coordination theory A. Verner
Building complex compounds
The interaction of particles is observed mutual coordination of particles, which can be defined as a process of complex formation. For example, the process of hydration of ions ends with the formation of aquacompleks. The complexation reactions are accompanied by the transfer of electronic pairs and lead to the formation or destruction of higher-order compounds, the so-called complex (coordination) compounds. A feature of complex compounds is the presence of a coordination bond resulting from a donor-acceptor mechanism:
Complex compounds are called compounds that exist both in crystalline state and in solution, feature
which is the presence of a central atom surrounded by ligands. Complex compounds can be considered as complex compounds of higher order consisting of simple molecules capable of independent existence in solution.

According to the coordination theory of Verner in the complex connection distinguish internaland external spheres.The central atom with the surrounding ligands form the inner sphere of the complex. It usually concludes in square brackets. Everything else in the complex compound is an external sphere and is written beyond square brackets. A certain number of ligands is stated around the central atom, which is determined. coordination number(CC). The number of coordinated ligands is most often equal to 6 or 4. Ligand occupies a coordination place near the central atom. When coordination, the properties of both ligands and the central atom are changed. Often, coordinated ligands cannot be detected using chemical reactions characteristic of them in a free state. More firmly related particles of the internal sphere are called complex (complex ion).Attraction forces act between the central atom and ligands (a covalent bond for exchange and (or) donor-acceptor mechanism is formed), between ligands - repulsion forces. If the charge of the internal sphere is 0, then the external coordination sphere is absent.
Central atom (complexing agent)- Atom or ion, which occupies the central position in the complex compound. The role of the complexing agent is most often performed by particles having free orbital and a rather large positive charge of the nucleus, and therefore can be electron acceptors. These are cations of transition elements. The strongest complexes are the elements of IB and VIIIB groups. Rarely as complex
retributors are neutral atoms of D-elements and non-metal atoms in varying degrees of oxidation. The number of free atomic orbitals provided by the complexing agent determines its coordination number. The value of the coordination number depends on many factors, but it is usually equal to the tweaking charge of an al-complexing agent:

Ligands- ions or molecules that are directly related to the complexing agent and are donors of electronic pairs. These electronic files having free and mobile electronic pairs can be electrons donors, for example:
Compounds of P-elements exhibit complexing properties and act in a comprehensive connection as ligands. Ligands may be atoms and molecules (protein, amino acids, nucleic acids, carbohydrates). According to the number of connections formed by ligands with a complex-educator, ligands are divided into mono-, di- and polydentate ligands.The above ligands (molecules and anions) are monoden-tatat, since they are donors of one e-pair. The bidentate ligands include molecules or ions containing two functional groups capable of being a donor of two electronic pairs:

Polydentate ligands include 6-dental ethylenediaminetetraacetic acid ligand:

The number of places occupied by each ligand in the inner sphere of a comprehensive compound is called coordination capacity (dentability) of the ligand.It is determined by the number of electronic pairs of ligand, which are involved in the formation of coordination communications with the central atom.
In addition to complex compounds, the coordination chemistry covers double salts, crystallohydrates disintegrating in an aqueous solution into composite parts, which in solid state in many cases are built in the same way as complex, but unstable.
The most stable and varied complexes in composition and the functions performed are formed by D-elements. Comprehensive compounds of transition elements are especially important: iron, manganese, titanium, cobalt, copper, zinc and molybdenum. Biogenic S -lements (Na, K, Mg, Ca) form comprehensive compounds only with ligands of a certain cyclic structure, speaking also as a complex agent. Main part r-Elements (N, P, S, O) is an active part of complex-forming particles (ligands), including bioligands. This consists of biological significance.
Consequently, the ability to complexization is the general property of the chemical elements of the periodic system, this ability decreases in the following order: f.> d.> p.> s.
7.2. Determination of the charge of the main particle compound
The charge of the inner sphere of the complex compound is an algebraic amount of charges of the particles forming it. For example, the value of the complex of the complex is determined as follows. Aluminum ion charge is +3, the total charge of six hydroxide ions -6. Consequently, the charge of the complex is equal to (+3) + (-6) \u003d -3 and the formula of the complex 3-. The charge of an integrated ion is numerically equal to the total charge of the external sphere and is opposite to him by sign. For example, the charge of the outer sphere K 3 is +3. Consequently, the charge of the complex ion is -3. The charge of the complex theater is equal in size and is opposite to the sign of the algebraic amount of charges of all other particles of the complex compound. From here, in K 3, the charge of iron ion is +3, since the total charge of all other particles of the complex compound is (+3) + (-6) \u003d -3.
7.3. Nomenclature of complex compounds
The foundations of the nomenclature are designed in the classic works of Werner. In accordance with them, in the complex compound, the cation is first called, and then anion. If the connection of non-electro-type type, then it is called in one word. The name of the complex ion is written into one word.
Neutral ligand is called the same as the molecule, and to the ligands anions are added at the end of "O". For a coordinated water molecule, the designation "Aqua" is used. To refer to the number of identical ligands in the inner sphere of the complex as a console before the ligand name, the Greek numeral di-, tri-, tetra, penta-, hexa-, etc. are used. Confix Mononux consumes. Ligands are listed in alphabetical order. The name of the ligand is considered as a whole. After the name of the ligand, the name of the central atom indicates the degree of oxidation, which is denoted by Roman numbers in parentheses. The word ammin (with two "M") is written in relation to ammonia. For all other amines, only one "M" is used.
C1 3 - hexamincobalt (III) chloride.
C1 3 - Aquapentamicobalt (III) chloride.
CL 2 - Pentamethylamminchlorocobalt (III) chloride.
Diammindibromoptiny (II).
If an integrated ion is an anion, then its Latin name has the end of "AM".
(NH 4) 2 - ammonium tetrachloropalladate (II).
K - Potassium Pentabromoamminplatinate (IV).
K 2 - Potassium Tetratodanadanobaltat (II).
The name of the complex ligand usually conclude in parentheses.
NO 3 - dichloro-di- (ethylenediamine) cobalt (III) nitrate.
Br - Bromo-tris- (triphenylphosphine) platinum (II) bromide.
In cases where the ligand binds two central ions, the Greek letter is used before its nameμ.

Such ligands are called bridgingand lists the last.
7.4. Chemical bond and structure of complex compounds
In the formation of complex compounds, the donor-acceptor interactions of the ligand and the central atom play an important role. The donor of the electronic pair is usually ligand. An acceptor is a central atom that has free orbital. This connection is durable and does not break when the complex is dissolved (Neoio-noun), and it is called coordination.
Along with the links, π-bonds for a donor-acceptor mechanism are formed. At the same time, the donor serves an ion of metal, gives off their paired D-electrons by ligand, which has energetically beneficial vacant orbital. Such connections are called dative. They are formed:
a) due to the overlapping of vacancies of metal p-orbitals with the D or-Batial Metal, on which there are electrons that have not entered into σ-communications;
b) when overlapping vacant Ligand D-orbital with filled metal D-orbital.
The measure of its strength is the degree of overlapping of the orbitals of the ligan and the central atom. The direction of bonds of the central atom determines the geometry of the complex. To explain the focus of links, the representations of the hybridization of atomic orbitals of the central atom are used. The hybrid orbitals of the central atom are the result of mixing uneven atomic orbitals, as a result, the form and energy of the orbital changes are mutually changed, and orbi-tali is formed by the same shape and energy. The number of hybrid orbitals is always equal to the number of source. Hybrid clouds are located in an atom at maximum distance from each other (Table 7.1).
Table 7.1.Types of hybridization of atomic orbitals of complex formation - la and geometry of some complex compounds

The spatial structure of the complex is determined by the type of hybridization of valence orbital and the number of vulnerable electronic pairs contained in its valence energy level.
The effectiveness of the donor-acceptor interaction of the ligand and the complexing agent, and consequently, the strength of the relationship between them (the stability of the complex) is determined by their polarizability, i.e. The ability to transform your electronic shells under external influence. On this basis, reagents are divided into "Hard",or lowolarizable and "Soft" -lightweight-rizuable. The polarity of the atom, molecule or ion depends on their size and number of electronic layers. The smaller the radius and electrons in the particle, it is less polarized. The smaller the radius and less electrons at the particle, the worse is polarized.
Rigid acids form with electric negative atoms O, N, F ligands (rigid bases) strong (rigid) complexes, and soft acids form with donor atoms P, S and I of ligands having low electronegativity and high polarizability, durable (soft) complexes. We see here a manifestation of the general principle "similar to the like".
Sodium ions, potassium due to their rigidity practically do not form sustainable complexes with biosubstrates and are in physiological environments in the form of aquacomplexes. Ca 2 + and Mg 2 + ions form quite stable complexes with proteins and therefore in physiological environments are both in ion and in the associated state.
D-elements ions are formed with biozubstrats (proteins) durable complexes. And soft acids CD, PB, HG are very toxic. They form durable complexes with proteins containing R-S Sulf-hydrogen groups:
Cyanide-ion toxic. Soft ligand actively interacts with D-metals in complexes with biosubstrates, activating the latter.
7.5. Dissociation of complex compounds. Stability of complexes. Labile and inert complexes
When dissolved in the water of complex compounds, they usually disintegrate into ions of external and internal spheres, similar to strong electrolytes, since these ions are associated ionically, mainly electrostatic forces. This is estimated as primary dissociation of complex compounds.
The secondary dissociation of the complex compound is the disintegration of the internal sphere to the components of its components. This process proceeds by the type of weak electrolytes, since the particles of the inner sphere are associated non-ionically (covalent bond). Dissociation is stepped in nature:

For the qualitative characteristic of the stability of the inner sphere of complex compound, the equilibrium constant is used, which describes the complete dissociation, called it, called constant of obstacity complex(KN). For complex anion - the expression of the constant of the obstacity has the form:

The smaller the KN value is, the more stable is the internal sphere of the complex compound, i.e. The less it dissociates in aqueous solution. Recently, instead of the KN, the value of the stability constant (KU) is the values, the return KN. The greater the value of ku, the more stable complex.
Sustainability constants make it possible to predict the direction of ligand exchange processes.
In an aqueous solution, the metal ion exists in the form of aquacomplexes: 2 + - hexaakvoryezo (II), 2 + - Tetraakvmed (II). When writing the formulas of hydrated ions, the coordinated water molecules of the hydrate shell do not indicate, but imply. The formation of a complex between the metal ion and in any ligand we consider as a reaction of substitution of the water molecule in the internal coordination sphere by this ligand.
The ligand exchange reactions proceed by the mechanism of reactions S n -typ. For example:
The values \u200b\u200bof the stability constants shown in Table 7.2 indicate that due to the complexation process, strong binding of ions occurs in aqueous solutions, which indicates the effectiveness of the use of this type of reactions for binding ions, especially polydentate ligands.
Table 7.2.Stability of zirconia complexes

In contrast to the reactions of ionic metabolism, the formation of complex compounds is often not a quasimegnaric process. For example, with the interaction of iron (III) with nitrilmetrythylene phosphonic acid, equilibrium is installed in 4 days. For the kinetic characteristics of complexes, concepts are used - labile(quickly reactive) and inert(slowly reactive). The labile complexes, at the proposal of the city of Taub, are considered to be equally exchanged by ligands for 1 min at room temperature and a solution concentration of 0.1 M. It is necessary to clearly distinguish the thermodynamic concepts [Durable (stable) / non-safe (unstable)] and kinetic [ Inert and labile] complexes.
In labile complexes, the replacement of ligands occurs quickly and quickly establishes equilibrium. In the inert complexes, the replacement of ligands flows slowly.
Thus, the inert complex 2 + in an acidic medium is thermodynamically unstable: the dismissed constant is 10 -6, and the labile complex is 2- very stable: the stability constant is 10 -30. The lability of Taube complexes associates with the electronic structure of the central atom. The inertness of complexes is characteristic mainly, ions with an unfinished D-shell. The inert includes complexes CO, CR. Cyanide complexes of many cations with an external level S 2 P 6 labils.
7.6. Chemical properties of complexes
The complexation processes affect almost the properties of all particles forming the complex. The higher the strength of the ligand bonds and the complexing agent, to the lesser extent, the properties of the central atom and ligands appear in the solution and the more noticeably the features of the complex affect.
Complex compounds show chemical and biological activity as a result of the coordination unsaturation of the central atom (there are free orbitals) and the availability of free electronic pairs of ligands. In this case, the complex has electro-filter and nucleophilic properties other than the properties of the central atom and ligands.
It is necessary to take into account the impact on the chemical and biological activity of the structure of the hydratic shell of the complex. The process of education
the complexes have an impact on the acid-main properties of a comprehensive compound. The formation of complex acids is accompanied by an increase in the force of an acid or base, respectively. So, in the formation of complex acids from simple binding energy with ions H +, the acid of acid is growing accordingly. If the ion is located in the outer sphere, the connection between the complex cation and the hydroxide ion of the external sphere is reduced, and the main properties of the complex are increasing. For example, copper hydroxide Cu (OH) 2 is a weak, hard-soluble base. Under the action of the ammonia, the ammonia of copper (OH) 2 is formed. The charge density is 2 + compared to Cu 2 + decreases, the connection with the ions it is weakened and (OH) 2 behaves like a strong base. The acid-basic properties of ligands associated with the complexing agent are usually manifested more strongly than the acid-basic properties of them in a free state. For example, hemoglobin (HB) or oxygemoglobin (HbO 2) exhibit acidic properties due to free carboxyl groups of protein-globin, which is a Ligand NNB ↔ H + + HB -. At the same time, the hemoglobin anion due to the amino group of the globin protein shows the basic properties and therefore binds acid oxide CO 2 to form an anion carbinamohemoglobin (NBSO 2 -): CO 2 + HB - ↔ NBSO 2 -.
Complexes show redox properties due to the redox transformations of the complex-educator forming sustainable oxidation degrees. The complexation process greatly affects the values \u200b\u200bof the reducing potentials of D-elements. If the reduced form of cations forms a more stable complex with this ligand than its oxidized form, then the potential value increases. The reduction in the amount of potential occurs when a more stable complex forms an oxidized form.For example, under the action of oxidizers: nitrites, nitrates, NO 2, H 2 O 2 hemoglobin as a result of the oxidation of the central atom turns into methemoglobin.
The sixth orbital is used in the formation of oxymemoglobin. The same orbital is involved in the formation of communication with carbon monoxide. As a result, a macrocyclic complex with iron - carboxygemoglobin is formed. This complex is 200 times more stable than the iron complex with oxygen in the heme.

Fig. 7.1.Chemical transformations of hemoglobin in the human body. Scheme from the book: Slyzarev V.I. Fundamentals of live chemistry, 2000
The formation of complex ions affects the catalytic activity of the ions of complexing agents. In some cases, activity increases. This is due to the formation of large structural systems in a solution capable of participating in the creation of intermediate products and reducing the activation energy of the reaction. For example, if KN 2 O 2 add Cu 2+ or NH 3, the decomposition process is not accelerated. In the presence of 2 + complex, which is formed in an alkaline medium, the decomposition of hydrogen peroxide accelerates 40 m30 times.
So, on hemoglobin, it is possible to consider the properties of complex compounds: acid-main, complexation and oxidative and recovery.
7.7. Classification of complex compounds
There are several classification systems for complex compounds that are based on various principles.
1. Applications of a comprehensive connection to a specific class of compounds:
Complex acids H 2;
Complex bases Oh;
Complex salts K 4.
2. By the nature of the Ligand: Akvakompleks, ammonias, acoxides (as ligands are anions of various acids, K 4; hydroxyl complexes (as ligands - hydroxyl groups, K 3); Complexes with macrocyclic ligands, inside which is located Central atom.
3. On the charge sign of the complex: cationic - complex cation in the complex compound Cl 3; anionic - complex anion in the complex compound k; Neutral - the charge of the complex is 0. The comprehensive compound of the external sphere does not have, for example. This is the formula of an antitumor preparation.
4. By the internal structure of the complex:
a) depending on the number of atoms of the complexing agent: mononuclear- the complex of the complex particle includes one atom of the complexing agent, for example, Cl 3; multi-core- As part of a complex particle, several atoms of the com-precipulator - the ironoprotein complex:

b) depending on the number of types of ligands distinguish complexes: homogeneous (one-grade),containing one type of ligand, for example 2 +, and heterogeneous (solid)- Two types of ligands or more, for example Pt (NH 3) 2 Cl 2. The complex includes Ligal-dying NH 3 and Cl -. For complex compounds containing various ligands in the inner sphere, it is characterized by geometric isomerism, when with the same composition of the inner sphere of ligands in it is located differently relative to each other.

Geometric isomers of complex compounds differ not only in physical and chemical properties, but also biological activity. Cis-isomer Pt (NH 3) 2 Cl 2 has pronounced antitumor activity, and the trans-isomer - no;
b) Depending on the dentability of ligands forming monooretric complexes, groups can be allocated:
Single-core complexes with monotental ligands, for example 3+;
Single-core complexes with polydentate ligands. Complex compounds with polydentate ligands call chelate compounds;
d) cyclic and acyclic forms of complex compounds.
7.8. Chelate complexes. Complexes. Comprehension
Cyclic structures that are formed as a result of the addition of metal ion to two donor atoms or more belonging to one molecule of the chelate-forming agent are called chelate compounds.For example, copper glycinate:

In them, the complexing agent seems to be inside the ligand, covered by bonds as culbs, so they, with other things being equal, have higher stability than compounds that do not contain cycles. The most stable cycles consisting of five or six links.This rule is first formulated by L.A. Chuhan. Difference
the stability of the chelate complex and the stability of its non-cyclic analog is called chelate effect.
As a chelating-forming agent, polydental ligands are served, which contain 2 types of groupings:
1) groups capable of the formation of covalent polar bonds due to the exchange reactions (protons donors, electronic pairs acceptors) -CH 2 Soam, -CH 2 PO (OH) 2, -CH 2 SO 2 OH, - acid groups (centers);
2) electronic pairs donors: ≡N,\u003e NH,\u003e C \u003d O, -S-, -OH, are the main groups (centers).
If such ligands are saturated with the internal coordination sphere of the complex and completely neutralize the charge of the metal ion, then the compounds are called intracomplex.For example, copper glycinate. There is no external sphere in this complex.
A large group of organic substances containing basic and acid centers in the molecule is called complex.These are polypic acids. Chelate compounds formed by complexones when interacting with metal ions, called complexons,for example, magnesium compounds with ethylenediaminetetraux acidic acid:
In an aqueous solution, the complex exists in anion form.
The complexons and complexons are a simple model of more complex compounds of living organisms: amino acids, polypeptides, proteins, nucleic acids, enzymes, vitamins and many other endogenous compounds.
Currently, a huge range of synthetic complexons with various functional groups is available. Formulas of the main complexons are presented below:


COMPUTERS under certain conditions can provide marginal electronic pairs (several) to form a coordination bond with a metal ion (S-, P- or D-element). As a result, steady compounds of chelate type with 4-, 5-, 6- or 8-membered cycles are formed. The reaction proceeds in a wide pH interval. Depending on the pH, the nature of the complexing agent, its ratio with the ligand is formed by complexons of various strength and solubility. The chemistry of complexation of complexons can be represented by equations on the example of an EDTA sodium salt (Na 2 H 2 Y), which dissociates in aqueous solution: Na 2 H 2 y → 2NA + + H 2 Y 2-, and ion H 2 Y 2- interacts with ions Metals Regardless of the oxidation of the metal cation, with one complex of the complexone interacts most often only one metal ion (1: 1). The reaction proceeds quantitatively (cr\u003e 10 9).
The complexons and complexons show in a wide range of pH amphoteric properties, the ability to participate in oxidation reactions, complexation, form compounds with a variety of properties, depending on the degree of metal oxidation, its coordination saturation, have electrophilic and nucleophilic properties. All this determines the ability to bind a huge number of particles, which allows the small number of reagent to solve large and varied tasks.
Another indisputable advantage of complexons and complexons is a small toxicity and ability to convert toxic particles.
in low-toxic or even biologically active. The products of the destruction of complexons are not accumulated in the body and harmless. The third feature of the complexonates is the possibility of their use as a source of trace elements.
Increased digestibility is due to the fact that the trace element is introduced in a biologically active form and has a high membrane-permeability.
7.9. Phosphoric metals complexons - an effective form of transformation of micro and macroelements into a biologically active state and a biological research model of chemical elements
Concept biological activitycovers a wide range of phenomena. From the point of view of chemical impacts under biologically active substances (BAV), it is customary to understand substances that can act on biological systems, regulating their livelihoods.
The ability to be interpreted as an ability to manifest biological activity. Regulation may manifest itself in the effects of stimulation, oppression, development of certain effects. The extreme manifestation of biological activity is biocidal actionwhen the exposure to the biocide substance, the latter dies. At lower concentrations, in most cases, biocides are not solid organisms, but a stimulating effect.
Currently, a large number of such substances are known. Nevertheless, in many cases, the use of famous BAV is used is not enough, often with efficiency, far from maximum, and the application often leads to side effects that can be eliminated by introducing modifier in the Bav.
Phosphorus-containing complexons form compounds with a variety of properties, depending on nature, the degree of metal oxidation, coordination saturation, composition and structure of the hydrate shell. All this determines the polyfunctionality of complex-nats, their unique ability of substroymometric action,
the effect of a common ion and provides widespread use in medicine, biology, ecology and in various sectors of the national economy.
When coordinating the metal ion of the complexone, electron density is redistributed. Due to the participation of radiating electronic pair with donor-acceptor interaction, the electronic density of the ligand (complex-n) to the central atom occurs. The decrease in the negative charge on ligand contributes to the reduction of the Coulomb repulsion of the reagents. Therefore, the coordinated ligand becomes more accessible to the attack of the nucleophilic reagent, which has an excess of electronic density at the reaction center. The displacement of the electron density from the complexion to the metal ion leads to a relative increase in the positive charge of the carbon atom, and therefore, to alleviate its attack with a nucleophilic reagent, hydroxyl ion. The hydroxylated complex among enzymes that catalyze metabolism processes in biological systems occupies one of the central places in the enzymatic action mechanism and the detoxification of the body. As a result of the multipoint interaction of the enzyme with the substrate, an orientation occurs, providing rapprochement of active groups in the active center and translation of the reaction to intramolecular mode, before the reaction and the formation of the transition state is started, which ensures the enzymatic FQM function.Conformational changes may occur in enzyme molecules. Coordination creates additional conditions for the redox interaction between the central ion and the ligand, since the immediate connection between the oxidizing agent and the reducing agent, which ensures the transition of electrons is established. For sets of transition metals, FQM can be characterized by the transitions of the electron-type L-M, M-L, M-L-M, in which orbitals both metal (M) and ligands (L) are involved, which are respectively associated with donor-acceptor bonds. Complexes can serve as a bridge for which the electrons of multi-core complexes are oscillating between the central atoms of one or different elements in varying degrees of oxidation (Electron and protons transfer complexes).The complexons determine the rehabilitation properties of metals complexons, which allows them to exhibit high antioxidant, adaptogenic properties, homeostatic functions.
So, the complexons convert trace elements into a biologically active form, available for the body. They form sustainable
more coordinated saturated particles, unable to destroy biocomplexes, and therefore, low-toxic forms. The complexons are favorable in violation of the microelement homeosta-beyond the organism. The transition ions in the complexonate form act in the body as a factor determining the high sensitivity of cells to microelements by their participation in creating a high concentration gradient, membrane potential. Components of transition metals FQM have bioregulatory properties.
The presence of acid and main centers in the composition of PCM provides amphoteric properties and their participation in maintaining acid-base equilibrium (isogide state).
With an increase in the number of phosphon groups in the composition of the complex, the composition and conditions for the formation of soluble and poorly soluble complexes are changed. An increase in the number of phosphon groups favors the formation of low-soluble complexes in a wider pH interval, shifts the area of \u200b\u200btheir existence in the acidic area. The decomposition of complexes occurs at a pH of more than 9.
The study of complexation processes with complexones made it possible to develop methods of bioregulators synthesis:
Stimulants of growth of prolonged action in colloid-chemical form are polygorean homo- and heterocompcesses of titanium and iron;
Growth stimulants in a water-soluble form. These are non-combined titanium compounds based on complexons and inorganic ligand;
Growth inhibitors - phosphorus-containing complexons of S-elements.
The biological effect of synthesized drugs on growth and development is studied in a chronic experiment on plants, animals and a person.
Bioregulation- This is a new scientific direction, which allows to regulate the direction and intensity of biochemical processes, which can be widely used in medicine, animal husbandry and crop production. It is associated with the development of ways to restore the physiological function of the body in order to prevent and treat diseases and age pathologies. Complexes and complex compounds based on them can be attributed to promising biologically active compounds. The study of their biological action in the chronic experiment showed that the chemistry gave into the hands of physicians,
livestock breeders, agronomists and biologists are a new promising agent, which allows to actively influence the living cell, regulate the power conditions, the growth and development of living organisms.
The study of the toxicity of used complexons and complexons showed the complete absence of the effects of drugs on the blood-forming organs, blood pressure, excitability, respiratory rate: no change in the liver function was noticed, a toxicological effect on the morphology of tissues and organs was not revealed. The potassium salt OEDF does not have toxicity in a dose, 5-10 times higher than therapeutic (10-20 mg / kg) during the study for 181 days. Consequently, complexones belong to low-toxic compounds. They are used as medicinal preparations to combat viral diseases, poisoning with heavy metals and radioactive elements, a violation of calcium metals, in endemic diseases and a violation of the balance of the trace element in the body. Phosphorus-containing complexes and complexons are not subjected to photolism.
The progressive pollution of the environment with heavy metals - products of human economic activity is an ever-effective environmental factor. They can accumulate in the body. The excess and disadvantage of them cause intoxication of the body.
Metal complexons, maintain in the body the chelating effect on the ligand (complex) and are indispensable for maintaining metal-rod homeostasis. The incorporated heavy metals are neutralized to a certain extent in the body, and the low resorption ability prevents the transmission of metals along the trophic chains, as a result, it leads to a certain "biomine system" of their toxic effect, which is especially true for the Ural region. For example, a free lead ion refers to thiol poisons, and a durable lead complex with ethylenediaminetetraacetic acid is low-oxidoxic. Therefore, the detoxification of plants and animals is to apply metals complexons. It is based on two thermodynamic principles: their ability to form strong bonds with toxic particles, turning them into poorly soluble or resistant connections in aqueous solution; Their inability to destroy endogenous biocompleks. In this regard, we consider it an important direction to combat the eco-ecorations and the production of environmentally friendly products - this complex therapy of plants and animals.
A study of the influence of plant treatment of complexons of various metals with intensive cultivation technology has been carried out.
potatoes on the microelement composition of potatoes. Poll samples contained 105-116 mg / kg of iron, 16-20 mg / kg of manganese, 13-18 mg / kg of copper and 11-15 mg / kg zinc. The ratio and content of trace elements are typical for vegetable tissues. Tubers grown using and without the use of metals complexons have almost the same elemental composition. The use of shela-com does not create conditions for the accumulation of heavy metals in the tubers. The complexons to a lesser extent than the metal ions are sorbed by the soil, resistant against its microbiological effects, which allows them for a long time to be held in the soil solution. Effect of 3-4 years. They are well combined with various pesticides. Metal in the complex has lower toxicity. Phosphorus-containing metals complexons are not irritated by the mucous membrane of the eye and do not damage the skin. Sensitizing properties are not detected, the cumulative properties of titanium complexons are not expressed, and some are very poorly expressed. The cumulation coefficient is 0.9-3.0, which indicates a low potential danger of chronic poisoning with drugs.
The basis of phosphorus-containing complexes is the phosphorrodic link (C-P), which is found in biological systems. It is part of phospho radipides, phosphonoglycans and phosphoproproteins of cell membranes. Lipids containing amino phosphonic compounds are resistant to enzymatic hydrolysis, ensure stability, and therefore, the normal functioning of outer cell membranes. Synthetic analogues of pyrophosphates - Diffos-Fones (R-C-P) or (R-C-C-P) in large doses violate calcium exchange, and in small normalize it. Diffosphonates are effective in hyperlipemia and promising from positions of pharmacology.
Diffosphonates, comprising P-C-P bonds, are structural elements of biosystems. They are biologically effective and are analogs of pyrophosphates. It is shown that diphosphonates are effective means of treating various diseases. Diphosphonates are active inhibitors of mineralization and bone resorption. The complexons convert trace elements into a biologically active shape, available for the body, form stable more coordination and saturated particles, unable to destroy biocomplexes, and consequently, low-toxic forms. They determine the high sensitivity of cells to trace elements, participating in the formation of a high concentration gradient. Able to participate in the formation of multi-core compounds of titanium heteroyantide
type - electrons and protons transfer complexes, participate in bioregulation of metabolic processes, organism resistance, ability to form connections with toxic particles, turning them into poorly soluble or soluble, stable, non-destructive endogenous complexes. Therefore, their use for detoxification, elimination from the body, obtaining environmentally friendly products (complexorates), as well as in industry for regeneration and utilization of industrial waste of inorganic acids and transition metal salts is very promising.
7.10. Ligand exchange and metal exchange
Equilibrium. Chelatotherapy
If there are several ligands with one metal ion or several metal ions with one ligand capable of forming complex compounds, competing processes are observed: in the first case, ligand exchange equilibrium is competition between metal ion ligands, in the second case, metal exchange equilibrium is competition between ions. Metal for ligand. The predominant will be the process of formation of the most solid complex. For example, in solution there are ions: magnesium, zinc, iron (III), copper, chromium (II), iron (II) and manganese (II). When introduced into this solution of a small amount of ethylenediaminetetraacetic acid (EDTA), competition between the metal ions and the binding to the iron complex (III) occurs, as it forms the most durable complex from EDTA.
The body constantly occurs in the interaction of biometals (MB) and biolygandov (LB), education and destruction of vital biocomplexes (MBLB):
In the body of man, animals and plants there are various mechanisms for the protection and support of this equilibrium from various xenobiotics (alien substances), including the ions of heavy metals. Heavy metal ions that are not associated with the complex, and their hydroxocomplexes are toxic particles (MT). In these cases, along with natural scaffolding, a new balance may occur, with the formation of more durable foreign complexes containing toxicant metals (MTLB) or ligands-toxicants (MBLT) that do not perform
required biological functions. If you get into the body of exogenous toxic particles, combined equilibrium arise and, as a result, competence of processes. The prevailing will be the process that leads to the formation of the most solid compound connection:

Metal-rigandous homeostasis disorders cause violations of the metabolism process, inhibit the activity of enzymes, destroy important metabolites, such as ATP, cell membranes, disturb the concentration gradient in cells. Therefore, artificial protection systems are created. Proper place in this method is occupied by chelate therapy (complex and therapy).
Chelatotherapy is the removal of toxic particles from the body, based on the chelating by their complexons of S-elements. Preparations used to eliminate the toxic particles incorporated in the body are called detoxicants(LG). The chelating of toxic particles of metals complexes (LG) converts toxic metal ions (MT) to non-toxic (MTLG) related forms suitable for insulation and penetration through membranes, transport and removal from the body. They retain in the body a chelating effect as a ligand (complex) and the metal ion. It provides metal-rigandous homeostasis of the body. Therefore, the use of complexons in medicine, animal husbandry, crop production ensures the detoxification of the body.
The main thermodynamic principles of chelate therapy can be formulated in two positions.
I. Detoxicant (LG) must effectively bind-toxicant ions (MT, Lt), newly formed compounds (MTLG) must be stronger than those that existed in the body:
II. Detoxicant should not destroy vital comprehensive compounds (MBLB); Compounds that can be formed in the interaction of the detoxicant and biometallic ions (MBLG) must be less durable than existing in the body:
7.11. The use of complexons and complexons in medicine
The complexomic molecules are practically not cleavage or any change in a biological environment, which is their important pharmacological feature. The complexons are insoluble in lipids and are well soluble in water, so they do not penetrate or penetrate well through cell membranes, and therefore: 1) are not output with the intestine; 2) the absorption of com-precipulating agents occurs only in their injection (only penicillamine is taken inward); 3) in the body complexes circulate by the advantage in the extracellular space; 4) the elimination of the body is carried out mainly through the kidneys. This process occurs quickly.
Substances that eliminate the effects of the effects of poisons on biological structures and inactivating poisons by chemical reactions are called antidote.
One of the first antidotes, which was applied in chelatotherapy is the British anti-lubrication (ball). Unitiol is currently used:
This drug effectively displays arsenic, mercury, chrome and bismuth from the body. The most widely used in the poisoning of zinc, cadmium, lead and mercury complexes and complexons. The use of them is based on the formation of more durable complexes with metal ions than complexes of the same ions with sulfur-containing groups of proteins, amino acids and carbohydrates. EDTA-based preparations are used to remove lead. Introduction to the body in large doses of drugs is dangerous, as they bind calcium ions, which leads to a violation of many functions. Therefore, use tetacin(Sana 2 EDTA), which is used to remove lead, cadmium, mercury, yttrium, cerium and other rare-earth metals and cobalt.

Since the first therapeutic use of Tetacin in 1952, this drug was widely used in the clinic of occupational diseases and continues to remain an indispensable antidote. The mechanism of action of the Tetacin is very interesting. Ion-toxicants displacing a coordinated calcium ion from thetaacin due to the formation of more durable bonds with oxygen and EDTA. Calcium ion, in turn, displaces two remaining sodium ions:

Thetacine is introduced into the body in the form of 5-10% solution, the basis of which is saline. Thus, after 1.5 hours after intraperitoneal injection, a 15% administered dose of thetaacin remains in the body, after 6 hours - 3%, and after 2 days - only 0.5%. The drug effectively and quickly, when applying the inhalation method of administration of thetaacin. It is quickly absorbed and circulates for a long time. In addition, Tetacin is used when protecting against gas gangrene. It inhibits zinc and cobalt ions, which are lecithinase enzyme activators, which is toxin gas gangrene.
The binding of toxicant tetacin into a small-toxic and more durable chelate complex, which is not destroyed and is easily excreted from the body through the kidneys, it provides detoxification and balanced mineral nutrition. Close in structure and composition for
eDTA's paratam is a sodium-calcium salt of diethylene-pentaucus acid (SANA 3 DTP) - pentatinand sodium salt diethylene pectantphosphonic acid (Na 6 DTPF) - trimph Qing.Pentazin is used mainly in poisoning with iron compounds, cadmium and lead, as well as to remove radionuclides (technetium, plutonium, uranium).

Sodium salt of ethyacid (SANA 2 Edtf) fousingsuccessfully used to eliminate mercury, lead, beryl, manganese, actinoids and other metals from the body. Complete complexes are very effective to remove some toxic anions. For example, cobalt (II) ethylene diameteetraacetate, forming a mixed-ligand complex with CN, can be recommended as an antidote during cyanide poisoning. A similar principle underlies the methods of removal of toxic organic substances, including pesticides containing functional groups with donor atoms capable of interacting with the metal of the complexonate.
Effective preparation is succimer(dimercastric acid, dimercaptosuccinic acid, hemet). It firmly connects almost all toxicants (HG, AS, PB, CD), but derives from the body of biogenic elements ions (CU, FE, Zn, CO), therefore it is almost no applied.
Phosphorus-containing complexons are powerful inhibitors of crystal formation of phosphates and calcium oxalates. As an anticalcifying drug in the treatment of urolithiasis, Xidihon is proposed - Caliyevo sodium salt OEDF. Diffosphonates, in addition, in minimum doses, increase the inclusion of calcium into bone tissue, prevent the pathological output from the bones. OEDF and other diphosphonates prevent various types of osteoporosis, including renal osteodistrophy, periodical
destruction, also destruction of transplanted bone in animals. The anti-seaterosclerotic effect of the EDF is also described.
In the United States, a number of dithosphonates were proposed, in particular EDF, as pharmaceutical preparations for the treatment of humans and animals suffering from metastasized bone cancer. Adjusting the permeability of membranes, diphosphonates contribute to the transportation of antitumor drugs into the cell, and hence the effective treatment of various oncological diseases.
One of the actual problems of modern medicine is the task of express diagnosis of various diseases. In this aspect, undoubted interest is a new class of drugs containing cations capable of performing the functions of the probe - radioactive magnetorecase and fluorescent tags. Radioisota of some metals are used as the main components of radiopharmaceutical preparations. The chelating of the cations of these isotopes of the complexes allows to increase their toxicological admissibility for the body, facilitate their transportation and ensure the selectivity of the concentration in certain bodies in certain limits.
The above examples do not exhaust the entire variety forms of the use of complexons in medicine. Thus, the diekal salt of magnesium ethylenediaminetethetate is used to regulate the content of fluid in tissues during pathology. EDTA is used in the composition of anticoagulant suspensions used in the separation of blood plasma, as a stabilizer of adenosine trifosphate in determining blood glucose, when lightening and storing contact lenses. Diffosphonates are widely used in the treatment of rheumatoid diseases. They are particularly effective as anti-pharmaceutical agents in combination with anti-inflammatory agents.
7.12. Complexes with macrocyclic compounds
Among the natural complex compounds, macrocomplexes based on cyclic polypeptides containing internal cavities of certain sizes are occupied, in which there are several oxygen-containing groups that can bind cations of those metals, including sodium and potassium, the dimensions of which correspond to the size of the cavity. Such substances while in biology

Fig. 7.2.Valinomycin complex with K + ion
materials, ensure the transport of ions through the membranes and therefore are called ionopors.For example, roliniumicin transports potassium ion through a membrane (Fig. 7.2).
With the help of another polypeptide - gramicidine a.sodium cations are transported by the relay mechanism. This polypeptide is rolled into the "tube", the inner surface of which is seduced by oxygen-containing groups. As a result, it turns out
a highly long hydrophilic channel with a certain cross section corresponding to the size of the sodium ion. Sodium ion, entering the hydrophilic channel on the one hand, is transmitted from one to other oxygen groups, like the relay on the ionically conductive channel.
So, the cyclic polypeptide molecule has an intramolecular cavity, which can enter a substrate substrate, geometry on the key principle and a lock. The cavity of such internal receptors is protected by active centers (endorecepto-rami). Depending on the nature of the metal ion, non-virulent interaction may occur (electrostatic, the formation of hydrogen bonds, Van der Wales forces) with alkaline metals and covalent with alkaline earth metals. As a result, they are formed supramolecu- Complex associates consisting of two particles or more held together by intermolecular forces.
The most common in the wilderness of the tetradentate macrocycles are Porphins and the corrinoids close to them.A schematic toreled cycle can be represented in the following form (Fig. 7.3), where the arcs mean the same type of carbon chains connecting donor nitrogen atoms into a closed cycle; R 1, R 2, R 3, P 4-hydrogen radicals; M N + - Metal ion: in chlorophyll ion Mg 2+, in hemoglobin Ion Fe 2+, in Hemocianin Ion Cu 2+, in Vitamin B 12 (Kobalamin) ion from 3+.
Nitrogen donor atoms are located at the corners of the square (indicated by dotted line). They are rigidly coordinated in space. therefore
porphyrins and corrinoids form solid complexes with cations of various elements and even alkaline earth metals. Essentially that regardless of the dentability of Ligand, the chemical bond and the structure of the complex are determined by donor atoms.For example, copper complexes with NH 3, ethylenediamine and pore-firin have the same square structure and similar electronic configuration. But polydentate ligands are associated with metal ions much stronger than monotentate ligands

Fig. 7.3.Tetradentate macrocycle
with the same donor atoms. The strength of ethylenediamine complexes by 8-10 orders more than the strength of the same metals with ammonia.
Biionorganic complexes of metal ions with proteins are called bioclathers -complexes of metal ions with macrocyclic compounds (Fig. 7.4).

Fig. 7.4.A schematic representation of the structure of bioclature of certain sizes of protein complexes with D-elements ions. Types of interactions of a protein molecule. M N + - Metal Ion Active Center
Inside the bioclaster there is a cavity. It includes metal, which interacts with the donor atoms of binding groups: it is, SH -, COO -, -NH 2, proteins, amino acids. The most famous metall farms
changes (carboangeerase, xanthine oxidase, cytochrome) are bioclasters, whose cavities form enzyme centers containing Zn, Mo, Fe, respectively.
7.13. Multi-core complexes
Heterovalent and heteroantore complexes
Complexes that include several central atoms of one or different elements, called multi-core.The possibility of the formation of multi-core complexes is determined by the ability of some ligands to bind to two or three ions of metals. Such ligands are called bridging.Respectively bridgingcustoms are called. Minds are also possible and monatomic bridges, for example:

They use essential electronic pairs belonging to the same atom. The role of bridges can perform multiatomic ligands.In such bridges, mixable electronic pairs belonging to different atoms are used. polyatomic ligand.

A.A. Greenberg and F.M. Filins investigated the bridging compounds of the composition in which the ligand binds the complex compounds of the same metal, but in various degrees of oxidation. Taube called them electron transfer complexes.It investigated the electron transfer reaction between the central atoms of various metals. Systematic studies of the kinetics and the mechanism of redox reactions led to the conclusion that the transfer of an electron between the two complexes of
it comes through the formed ligand bridge. The electron exchange between 2 + and 2 + occurs through the formation of the intermediate bridge complex (Fig. 7.5). The electron transfer occurs through chloride bridge ligand, ending with the formation of 2 + complexes; 2 +.

Fig. 7.5.Electron transfer in an intermediate multi-core complex
A wide variety of polyderous complexes was obtained through the use of organic ligands containing several donor groups. The condition for their formation is the location of donor groups in a ligand, which does not allow to close the chelate cycles. There are no cases when the ligand has the ability to close the chelate cycle and at the same time act as a bridge-owned.
The current start of the transfer of the electron are transition metals that show several stable oxidation degrees. This gives titanium, iron and copper ions. The perfect properties of electron carriers. The combination of options for the formation of hetero-tanny (GVK) and heteronuclear complexes (GIK) based on Ti and Fe are presented in Fig. 7.6.
Reaction

Reaction (1) is called cross reaction.In metabolic reactions, the intermediates will be heterovalent complexes. All theoretically possible complexes are truly formed in solution in certain conditions, which has been proven by various physico-chemical

Fig. 7.6.The formation of heterobler complexes of heteroantore complexes containing Ti and Fe
methods. To carry out the transfer of electrons, the reagents must be in the energies of states. This requirement is called the principle of Frank Condon. The electron transfer can occur between the atoms of one transition element in different degrees of the oxidation of GVK, or the various elements of the gayak, the nature of metallocene, which is different. These compounds can be defined as electrons transfer complexes. They are convenient carriers of electrons and protons in biological systems. The attachment and return of the electron causes changes only to the electronic configuration of the metal, without changing the structure of the organic component of the complex.All these elements have several stable oxidation degrees (Ti +3 and +4; Fe +2 and +3; Cu +1 and +2). In our opinion, these systems are provided by nature a unique role of ensuring the reversibility of biochemical processes with minimal energy costs. Reversible reactions include reactions having thermodynamic and thermochemical constants from 10 -3 to 10 3 and with a slight value of ΔG O and E O.processes. In these conditions, the initial substances and reaction products can be in commensurate concentrations. When they change in some range, it is easy to achieve reversibility of the process, therefore, in biological systems, many processes are oscillatory (wave). Redox systems that have the above pairs are overlapped by a wide range of potentials, which allows them to enter into interactions, accompanied by moderate changes Δ G O.and E °, with many substrates.
The probability of the formation of GVK and GIK is significantly increasing when the solution contains potentially bridged ligands, i.e. Molecules or ions (amino acids, hydroxyc acid, complexons, etc.), capable of tying two metallo center at once. The possibility of delocalizing an electron in GVK contributes to a decrease in the total energy of the complex.
A more realistic set of possible options for the formation of GVK and GIK, in which the nature of metal centers is different, visible in Fig. 7.6. A detailed description of the formation of GVK and Gaik and their role in biochemical systems are considered in the works of A.N. Glebova (1997). Redox couples should be structurally adjusted to each other, then the transfer becomes possible. Selecting the components of the solution, you can "lengthen" the distance to which the electron from the reducing agent is transferred to the oxidizer. With a consistent movement of particles, the electron transition can occur over long distances along the wave mechanism. As a "corridor" can be a hydrated protein chain and others. High probability of electron transfer by distance to 100a. The length of the "corridor" can be increased by additives (alkali metal ions, background electrolytes). This opens up great opportunities in the field of management of the composition and properties of GVK and Gaik. In solutions, they play the role of a kind of "black box" filled with electrons and protons. Depending on the circumstances, it can give them to other components or replenish its "stocks". The reversibility of reactions with their participation allows multiple times to participate in cyclic processes. Electrons go from one metal center to another, oscillate between them. The complex molecule remains asymmetric and can take part in oxidative and rehabilitation processes. GVK and Gyak are actively involved in vibrational processes in biological environments. This type of reaction is called oscillatory reactions.They found in enzymatic catalysis, synthesis of proteins and other biochemical processes associated with biological phenomena. This includes periodic processes of cell metabolism, waves of activity in cardiac tissue, in brain tissue and processes occurring at the level of environmental systems. An important step of metabolism is the cleavage of hydrogen from nutrients. The hydrogen atoms are moving into the ionic state, and the electrons separated from them enter the breathing chain and give their energy to the formation of ATP. As we have installed, titanium complexes are active carriers not only electrons, but also protons. The ability of titanium ions to fulfill its role in the active center of catalase type enzymes, peroxidase and cytochromes is determined by its high ability to complexation, the formation of a coordinated ion geometry, the formation of multi-core GVK and GIK of various composition and properties in the function pH, concentration of the transition element Ti and the organic component of the complex, their molar ratio. This ability is manifested in improving the selectivity of the complex
in relation to substrates, products of metabolic processes, activation of links in a complex (enzyme) and a substrate by coordination and changes in the form of a substrate in accordance with the sterhing demands of the active center.
The electrochemical transformation in the body associated with the transfer of electrons is accompanied by a change in the degree of oxidation of particles and the occurrence of the oxidation and reduction potential in the solution. A large role in these transformations belongs to multi-core complexes of GVK and GIK. They are active regulators of free radical processes, the utilization system of the active forms of oxygen, hydrogen peroxide, oxidizing agents, radicals and are involved in the oxidation of substrates, as well as in maintaining antioxidant homeostasis, in protecting the body from oxidative stress.Their enzymatic action on the biosystems is similar to enzymes (cytochro-mothers, superoxiddismutaz, catalase, peroxidase, glutathione-reductase, dehydrogenases). All this indicates the high antioxidant properties of transitional elements complexons.
7.14. Questions and tasks for self-testing of training for classes and exams
1. The concept of complex compounds. What is their difference from double salts, and what do they have in common?
2. Come on the formula of complex compounds according to their name: ammonium dihydroxotetrachloropottinate (IV), triammmingrinitro-balt (III), give them a characteristic; Specify the inner and external coordination sphere; Central ion and its oxidation degree: ligands, their number and dentability; The nature of connections. Write dissociation equation in aqueous solution and expression for stability constant.
3. General properties of complex compounds, dissociation, stability of complexes, chemical properties of complexes.
4. How does the reactivity of the complexes characterizes with thermodynamic and kinetic positions?
5. What amino acids will be more durable than tetraamino-copper (II), and what are less durable?
6. The example examples of macrocyclic complexes formed by alkali metal ions; D-elements ions.
7. What sign do the complexes refer to chelate? Give examples of chelate and non-fermented complex compounds.
8. In the example of glycinat copper, give the concept of intracomplex connections. Write a structural formula of magnesium complexomation with ethylenediaminetetraacetic acid in sodium form.
9. Schedule a schematic fragrant fragment of any polyders complex.
10. Let the definition of polynuclear, heteroantore and hetero-tape complexes. The role of transition metals in their formation. The biological role of the data of the components.
11. What types of chemicals are found in integrated with unity?
12. Transfer the main types of hybridization of atomic orbitals, which may occur at the central atom in the complex. What is the geometry of the complex depending on the type of hybridization?
13. What comes from the electronic structure of atoms of elements S-, P- and D-blocks to compare the ability to complexation and their place in the chemistry of complexes.
14. Let the definition of complexons and complexons. Give examples of the most used in biology and medicine. Bring the thermodynamic principles on which chelatotherapy is based. The use of complexons for neutralization and elimination of xenobiotics from the body.
15.Cide the main cases of impaired metal and human homeostasis in the human body.
16. For examples of biocomplex compounds containing iron, cobalt, zinc.
17. Examples of competing processes with the participation of hemoglobin.
18.Rol Metal ions in enzymes.
19. Possible why for cobalt in complexes with complex ligands (polydentate) is more resistant to oxidation +3, and in conventional salts, such as halides, sulfates, nitrates, oxidation degree +2?
20.The Copper is characterized by oxidation degrees +1 and +2. Can copper catalyze reactions with electron transfer?
21. Can zinc catalyze redox reactions?
22. What is the mechanism of action of mercury as poison?
23. For the acid and the base in the reaction:
AGNO 3 + 2NH 3 \u003d NO 3.
24. Practice why the drug-sodium salt of hydroxyethylidendythosphonic acid is used as a medicinal drug, and not OEDF.
25.Cax with metal ions included in biocomplex connections, electrons transported in the body?
7.15. Test tasks
1. The degree of oxidation of the central atom in the complex ion 2- equal to:
a) -4;
b) +2;
at 2;
d) +4.
2. The most steady complex ion:
a) 2-, kn \u003d 8,5х10 -15;
b) 2-, kn \u003d 1.5x10 -30;
c) 2-, kn \u003d 4x10 -42;
d) 2-, kn \u003d 1x10 -21.
3. The solution contains 0.1 mol of PTCl 4 4NH 3 compound. Responding to AGNO 3, it forms 0.2 mol of AGCL sediment. Remove the initial substance coordination formula:
a) cl;
b) Cl 3;
c) Cl 2;
d) Cl 4.
4. What form are complexes formed as a result sP 3 D 2- brdise?
1) tetrahedra;
2) square;
4) trigonal bipiramid;
5) Linear.
5. Pickup the formula for compound PentammammocularBalt (III) sulfate:
a) Na. 3 ;
6) [SL 2 (NH 3) 4] CL;
c) K 2 [CO (SCN) 4];
d) SO 4;
e) [co (n 2 O) 6] C1 3.
6. What ligands are polydentate?
a) C1 -;
b) H 2 O;
c) ethylenediamine;
d) NH 3;
e) SCN -.
7. The complexing agents are:
a) atoms donors of electronic pairs;
c) atoms and ions-acceptors of electronic pairs;
d) atoms and donor ions of electronic pairs.
8. The smallest complex-forming ability elements possess:
a) s; c) d;
b) P; d) F.
9. Ligands are:
a) electronic pairs donor molecules;
b) electronic pairs acceptor ions;
c) molecules and ion donors of electronic pairs;
d) molecules and ions - electronic pairs acceptors.
10. Communication in the internal coordination sphere of the complex:
a) covalent exchange;
b) covalent donor-acceptor;
c) ionic;
d) hydrogen.
11. The best complexeer will be:
Comprehensive compounds. Their structure based on the coordination theory of A. Verner. Complex ion, his charge. Cationic, anionic, neutral complexes. Nomenclature, examples.



Reactions replacement of ligands. Constant of unstable complex ion, stability constant.

 The ratio of the concentration of broken ions to the unprecedented quantity is the ratio of the concentration of unprecedented ions.
The ratio of the concentration of broken ions to the unprecedented quantity is the ratio of the concentration of unprecedented ions.
To the mouth \u003d 1 / to Nest (inverse)
 Secondary dissociation -the disintegration of the internal sphere of the complex to the components components.
Secondary dissociation -the disintegration of the internal sphere of the complex to the components components.

43. Consection for the ligand or for the complexing agent: isolated and combined equilibrium replacement of ligands. The total constant of the alignment equilibrium of the substience of ligands.
As a result of competition, the proton destroys a rather durable complex, forming a weak dissociator - water.
CL + NIS0 4 + 4NH 3 ^ S0 4 + AGCL i
This is an example of a ligand competition for the complexing agent, with the formation of a more durable complex (KH + \u003d 9.3-1 (g 8; K H [M (W 3) 6] 2+ \u003d 1.9-Yu-9) and an employment compound AgCl - k s \u003d 1.8 10 "10
Representations on the structure of metal farms and other biocomplex compounds (hemoglobin, cytochrome, cobalamins). Physico-chemical principles of oxygen transport hemoglobin





Cobalamins. Vitamins B 12. They call a group of cobalt-containing biologically active substances called cobalamins. These include actually cyanocobalamin, Hydroxycobalamin and two coenses of vitamin B 12: methylcoobalamine and 5-deoxyadenosylcobalamine.
Sometimes in a narrower sense, vitamin B 12 is called cyanocobalamine, since it is precisely in this form to the human body the main amount of vitamin B 12 is received, without losing the fact that it is not synonymous with B 12, and several other compounds also possess B 12 - vitamin activity. Vitamin B 12 is also called the outer factor of Castle.
B 12 has the most complex chemical structure compared to other vitamins, the basis of which is the Corrinoecolo. Corrin is in many ways similar to Porphyrin (complex chemical structure, which is part of the hem, chlorophyll of icotochromes), but differs from the porphyrin in the fact that the two pyrrolean cycles in the corrod are interconnected directly, and not methylene bridge. In the center of the Corrinic structure is the cobalt ion. Four coordination bonds cobalt forms with nitrogen atoms. Another coordination relationship connects cobalt with a coinmetythylbenzimidazole nucleotide. The latter, the sixth coordination link of cobalt remains free: it is for this connection that a cyano group, a hydroxyl group, a methyl or 5 "-deoxyadenosal residue, with the formation of four variants of vitamin B 12, is joined, respectively. Covalent carbon-cobalt connection in the structure of cyanocobalamin - the only known alive Nature Example of covalent bond transition metal-carbon.
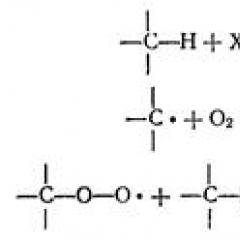
The main reaction of substitution in aqueous solutions is the exchange of water molecules (22) - was studied for a large number of metal ions (Fig. 34). The exchange of water molecules of the coordination sphere of metal ion with the main mass of water molecules present as a solvent, for most metals proceeds very quickly, and therefore the velocity of such a reaction was carried out mainly by relaxation. The method consists in violation of the equilibrium of the system, for example a sharp increase in temperature. Under new conditions (higher temperatures), the system will no longer be in equilibrium. Then measure the rate of establishment of equilibrium. If you can change the temperature of the solution during 10 -8 secondsthen you can measure the reaction rate that requires more time for its completion 10 -8 seconds.
It is also possible to measure the rate of substitution of coordinated water molecules in various metal ions with ligands SO 2- 4, S 2 O 3 2-, EDTA, etc. (26). The speed of such a reaction
it depends on the concentration of hydrated metal ion and does not depend on the concentration of the incoming ligand, which allows the first order equation to describe these systems (27). In many cases, the reaction rate (27) for this metal ion does not depend on the nature of the incoming ligand (L), whether it is H 2 O molecules or ions SO 4 2-, S 2 O 3 2- or EDTA.
This observation, as well as the fact that the concentration of the incoming ligand is not included in the speed equation of this process, suggest that these reactions proceed by the mechanism in which the slow stage is to break the connection between the metal ion and water. The resulting connection is likely to then quickly coordinates the ligands nearby.
In section. 4 of this chapter was indicated that higher hydrated metal ions, such as Al 3+ and SC 3+, exchange water molecules slower than m 2+ and M + ions; This gives reason to assume that in the stage determining the speed of the entire process, the rupture of connections plays an important role. The conclusions obtained in these studies are not final, but they give reason to think that in the reactions of substitution of hydrated metals ions, S n 1-processes are important.
Probably the most studied complex compounds are Cobalt (III) amines. Their stability, ease of preparation and slowly current reactions with them make them particularly comfortable for kinetic studies. Since the studies of these complexes were carried out exclusively in aqueous solutions, initially consider the reactions of these complexes with solvent molecules - water. It was found that in general the ammonia or amine molecules, coordinated by the CO (III) ion, are so slowly replaced by water molecules, which usually consider the replacement of other ligands, and not amines.
The speed of type (28) reactions was studied and it was found that it was first about the cobalt complex (X is one of the many possible anions).
Since in aqueous solutions, the concentration of H 2 O is always equal to about 55.5 M.It is impossible to determine the effect of changes in the concentration of water molecules on the reaction rate. The speed equations (29) and (30) for an aqueous solution are not experimentally distinguishable, since it is simply equal to k "\u003d k". Consequently, the reaction rate equation cannot be said whether H 2 O participate in the stage determining the speed of the process. The answer to the question is whether this reaction is under the mechanism S n 2 with the replacement of ion X on the H 2 O molecule or by the mechanism S N 1, which first envisages the dissociation, followed by the addition of the H 2 O molecule, must be obtained using other experimental data.
Solutions of this task can be achieved by two types of experiments. The speed of hydrolysis (replacement of one CL ion - on the water molecule) trance- + approximately 10 3 times the speed of hydrolysis 2+. The increase in the charge of the complex leads to an increase in the ties of the metal - ligand, and consequently, to the braking of the discontinuity of these connections. It should also be taken into account the attraction of incoming ligands and facilitating the flow of reaction reaction. Since a decrease in speed is detected as the charge of the complex increases, then in this case it seems a more likely dissociative process (S N 1).
Another way of evidence is based on the study of hydrolysis of a series of complexes of similar trance- +. In these complexes, the ethylenediamine molecule was replaced with similar diamines in which hydrogen atoms at the carbon atom are substituted in groups CH 3. Complexes containing substituted diamines react faster than the ethylene diamine complex. The replacement of hydrogen atoms on CH 3-group increases the volume of the ligand, which makes it difficult to attack the metal atom by another ligand. These sterile obstacles slow down the reaction according to the mechanism S N 2. The presence of the volume atom of the bulk ligand near the metal atom contributes to the dissociative process, since the removal of one of the ligands reduces their accumulation at the metal atom. The observed increase in the hydrolysis rate of complexes with bulk ligands is a good proof of the reaction flow through the mechanism S N 1.
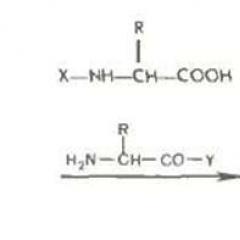
So, as a result of numerous studies of the acidine amine complexes CO (II), it turned out that the replacement of acid agroups of water molecules is a dissociative process in its nature. The connection of the cobalt atom - ligand is extended to some critical value before the water molecules begin to enter the complex. In the complexes that have a charge 2+ and above, the tile of communication cobalt - ligand is very difficult, and the entry of water molecules begins to play a more important role.
It was found that the replacement of the Acido group (x -) in the cobalt (III) complex to another group than the H 2 O molecule (31) passes at first through the substitution of its molecule
solvent - water with subsequent replacement for its new group Y (32).
Thus, in many reactions with cobalt complexes (III), the reaction rate (31) is equal to the hydrolysis rate (28). Only hydroxyl ion differs from other reagents with respect to reactivity with Ammines Co (III). It reacts very quickly to the ammous complexes of cobalt (III) (approximately 10 6 times faster than water) by the type of reaction basic hydrolysis (33).
It was found that this reaction of the first order relative to the replacement Ligand OH - (34). The total second reaction procedure and unusually rapid flow of reaction suggest that ion OH is an exceptionally effective nucleophilic reagent with respect to CO (III) complexes and that the reaction proceeds through the mechanism S n 2 through the formation of an intermediate connection.
However, this property OH can also be explained by another mechanism [equations (35), (36)]. In reaction (35) Complex 2+ behaves like an acid (according to Brenets), giving a complex +, which is amido- (containing) -to-compound - the base corresponding to acid 2+.
The reaction then flows through the mechanism S n 1 (36) to form a five-coordinate intermediate, further reacting with solvent molecules, which leads to the final reaction product (37). This reaction mechanism is consistent with the second-order response rate and corresponds to the mechanism S n 1. Since the reaction in the stage determining the rate includes the base involved in the initial complex - acid, then this mechanism is given the designation S n 1SV.
To determine which of these mechanisms is best explaining experimental observations, it is very difficult. However, there are convincing evidence confirming the hypothesis S N 1CB. The best arguments in favor of this mechanism are the following: Octahedral complexes of CO (III) at all react at the dissociative mechanism Sn 1, and there are no convincing arguments, why would OH ion - should determine the process Sn 2. It has been established that the hydroxyl ion is a weak nucleophilic reactant in reactions With Pt (II), and therefore its unusual reactivity with respect to CO (III) seems to be unreasonable. Reactions with cobalt (III) compounds in non-aqueous media serve as excellent proof of the formation of five-coordinate intermediate compounds provided for by the mechanism of S N 1 SV.
The final proof is the fact that, in the absence of CO (III), the N-H connections in the CO (III) reacts with ions it -. This, of course, gives reason to believe that for the reaction rate of the acid-basic properties of the complex is more important than nucleophilic properties, it. This reaction of the main hydrolysis of amine complexes CO (III) is an illustration of the fact that the kinetic data can often be interpreted not only in one way, and To eliminate one or another possible mechanism, you need to make a rather thin experiment.
Currently, the reaction of replacement of a large number of octahedral compounds is investigated. If we consider their reaction mechanisms, then the dissociative process is most often found. This result is not unexpected, since six ligands leave little space around the central atom to attach other groups to it. It is known only to some examples when the occurrence of a seven-coordinate intermediate compound is proved or the influence of the introduced ligand is detected. Therefore, S n 2, the mechanism cannot be fully rejected as a possible path of reactions of substitution in octahedral complexes.
Introduction to work
Relevance of work. Porphyrin complexes with metals in high degrees of oxidation can coordinate the bases much more efficiently than M 2+ complexes and form mixed coordination compounds in which in the first coordination sphere of the central metal atom, along with the macrocyclic ligand, are non-cyclic acidoligans, and sometimes coordinated molecules. The compatibility issues of ligands in such complexes are extremely important, since it is precisely in the form of mixed complexes of porphyrins carry out their biological functions. In addition, the reaction of reversible addition (transfer) of the base molecules characterized by moderately high equilibrium constants can be successfully used to separate mixtures of organic isomers, for quantitative analysis, for the purposes of ecology and medicine. Therefore, studies of quantitative characteristics and stoichiometry of equilibrium of additional coordination on metal-mills (MR) and substitution of simple ligands in them are useful not only from the point of view of theoretical knowledge of the properties of metallofirins as compounds of complex, but also to solve the practical task of finding receptors and carriers of small molecules or ions. To date, systematic studies for high-passing metal ions are practically absent.
purpose of work. This paper is devoted to the study of the reactions of mixed porphyrine-containing complexes of high-passing cations of Metals ZR IV, HF IV, MO V and WV with bioactive N-bases: imidazole (IM), pyridine (PY), pyrazine (PYZ), benzimidazole (BZIM), characteristic Stability and optical properties of molecular complexes, justification of stepped reaction mechanisms.
Scientific novelty. The methods of modified spectrophotometric titration, chemical kinetics, electron and oscillatory absorption and 1 H NMR spectroscopy were first obtained by thermodynamic characteristics and the stoichiometric mechanisms of N-base reactions with metalloporphyrins with a mixed coordination sphere (x) N-2 MTRR (x - acidoligand CL -, OH -, O 2-, TRP - Tetraphenylporpirin dyanion). It has been established that in the overwhelming majority of cases, the processes of formation of supramolecules metalloforphyrin - the base flows stepwise and includes several reversible and slow irreversible elementary reactions of coordination of the base molecules and replacement of acidoligands. For each of the steps of step reactions, stoichiometry, equilibrium constants or speed, the orders of slow reactions on the base, spectrally characterized products (UV, visible spectra for intermediate products and UV, visible and IR - for the end). For the first time, correlation equations were obtained to predict the stability of supramolecular complexes with other bases. The equations are used in operation to discuss the detailed substitution mechanism, it is in MO and W complexes on the base molecule. The properties of MR are described, which cause the prospect of use for detecting, separation and quantitative analysis of biologically active bases, such as moderately high sustainability of supramolecular complexes, a clear and fast optical response, low sensitivity threshold, second time.
Practical significance of work. Quantitative results and substantiation of stoichiometric mechanisms of molecular complexation reactions are essential for the coordination chemistry of macrohyterocyclic ligands. The dissertation is shown that mixed porphyrin-containing complexes show high sensitivity and selectivity for bioactive organic bases, within a few seconds or minutes give an optical response, suitable for practical detection of reactions with bases - VOCs, components of drugs and food products, due to Recommended for use as components of base sensors in ecology, food industry, medicine and agriculture.
Approbation of work. The results of the work were reported and discussed on:
IX International Conference on Solvation and Collecting Complexation in solutions, Ples, 2004; XII symposium for intermolecular interaction and conformations of molecules, Pushchino, 2004; XXV, XXVI and XXIX scientific sessions of the Russian seminar on the chemistry of porphyrins and their analogues, Ivanovo, 2004 and 2006; VI School-conference of young scientists of the CIS countries in the chemistry of porphyrins and related compounds, St. Petersburg, 2005; VIII Scientific School - Conferences on Organic Chemistry, Kazan, 2005; All-Russian scientific conference "Natural macrocyclic compounds and their synthetic analogues", Syktyvkar, 2007; XVI International Conference on Chemical Termodynamics in Russia, Suzdal, 2007; XXIII International Chughaev Conference on Coordination Chemistry, Odessa, 2007; International Conference On Porphyrins and Phtalocyanines ISPP-5, 2008; 38th International Conference On Coordination Chemistry, Israel, 2008.
Conditionally chemical reactions of the complexes are divided into exchange, oxidative, rehabilitation, isomerization and coordinated ligands.
The primary dissociation of complexes on the inner and outer sphere determines the flow of the exchange of expenditure ions:
X M + MNAY \u003d Y M + MNAX.
The components of the internal sphere of complexes can also participate in metabolic processes with the participation of both ligands and the complexing agent. To characterize the reactions of substitution of ligands or the central metal ion, the designations and terminology proposed by K. Ingold for the reactions of organic compounds are used (Fig. 42), nucleophilicS N. and electrophileS e substitution:
Z + y \u003d z + x s n
Z + m "\u003d z + m s e.
According to the reaction mechanism, the substitution is divided (Fig. 43) to the associative (S N 1 and S E 1 ) and dissociative (S N 2 and S E 2 ), differing in transition with an enlarged and reduced coordination number.
The assignment of the reaction mechanism to associative or dissociative is difficult to experimentally achieve the task of identifying an intermediate with a reduced or increased coordination number. In this regard, often the mechanism of the reaction is judged on the basis of indirect data on the effect of the concentration of reagents on the reaction rate, change in the geometric structure of the reaction product, etc.
To characterize the rate of reactions of reactions of ligands of complexes, the Nobel laureate of 1983 G. Taube (Fig. 44) proposed to use the terms "labile" and "inert", depending on the time of the reaction of the reaction of ligands less than or more than 1 minute. The terms labile or inert are the characteristic of the kinetics of reactions of ligand reactions and they should not be confused with the thermodynamic characteristics of the stability or nonstability of the complexes.
The lability or inertness of the complexes depends on the nature of the ion of the complexing agent and ligands. In agreement with the theory of the field of ligands:
1. Octahedrian complexes 3.d. transition metals with valence distribution (n -1) D electrons on Sigma* (E G ) Breakdown Mo labils.
4- (T 2G 6 E G 1) + H 2 O= 3- + CN -.
Moreover, the less the energy of the stabilization of the crystal field of the complex, the lability is greater.
2. Octahedrian complexes 3.d. transition metals with free sigma* tearing E G orbitals and uniform distribution of valence (n -1) D electrons by t 2 g orbitals (T 2 G 3, T 2 G 6) are inert.
[CO III (CN) 6] 3- (T 2 G 6 E G 0) + H 2 O \u003d
[CR III (CN) 6] 3- (T 2 G 3 E G 0) + H 2 O \u003d
3. Flat-square and octahedral 4d and 5 D transition metals that do not have electrons on sigma* Breakdown Mo inert.
2+ + H 2 O \u003d
2+ + H 2 O \u003d
The influence of the nature of ligands on the rate of reaction reactions of ligands is considered within the framework of the "mutual influence of ligands". A special case of the model of mutual influence of ligands is, formulated in 1926 I.I. Chernyaev the concept of trans-influence (Fig. 45) - "The lability of ligand in the complex depends on the nature of the trans-located ligand" - and offer a number of trans-influences of ligands:CO, CN -, C 2 H 4\u003e PR 3, H -\u003e CH 3 -, SC (NH 2) 2\u003e C 6 H 5 -, NO 2 -, I -, SCN -\u003e BR -, CL -\u003e PY , NH 3, OH -, H 2 O.
The concept of trans-influence made it possible to substantiate empirical rules:
1. Painone rule - under the action of ammonia or amines on the tetrachlopla-tinat (II. ) Potassium is always dichlodiamineplatin Cis-configuration:
2 - + 2NH 3 \u003d CIS - + 2CL -.
Since the reaction proceeds into two stages and chloride ligand has a large trans influence, the substitution of the second chloride ligand on ammonia occurs with the formation of cis- [Pt (NH 3) 2 Cl 2]:
2- + NH 3 \u003d -
NH 3 \u003d cis -.
2. Rule Hiergensen - under the action of hydrogenic acid on the chloride of Tetrambammina Platinum (II. ) or similar compounds obtained dichlorod-amminplatin trans-configuration:
[Pt (NH 3) 4] 2+ + 2 HCl \u003d trans- [pt (NH 3) 2 Cl 2] + 2 NH 4 Cl.
In accordance with a number of trans-influence of ligands, the replacement of the second ammonia molecule on chloride ligand leads to the formation of trans-Pt (NH 3) 2 Cl 2].
3. Tiomea reaction Kurnakova - Various products of Tiomo-Chevina reaction with geometric trans- measurements [Pt (NH 3) 2 Cl 2] and cis- [PT (NH 3) 2 Cl 2]:
cis - + 4thio \u003d 2+ + 2Cl - + 2NH 3.
The different nature of the reaction products is associated with the high trans-influence of thiourevine. The first stage of reactions is the substitution of thiochemical chloride ligands with the formation of trans- and cis- [Pt (NH 3) 2 (THIO) 2] 2+:
trans- [PT (NH 3) 2 Cl 2] + 2 thio \u003d trans- [pt (NH 3) 2 (THIO) 2] 2+
cis - + 2thio \u003d cis - 2+.
In cis- [pt (NH 3) 2 (thio ) 2] 2+ Two ammonia molecules in trans position to thiourevine are subjected to further substitution, which leads to education 2+ :
cis - 2+ + 2thio \u003d 2+ + 2NH 3.
In the trans- [PT (NH 3) 2 (thio ) 2] 2+ Two ammonia molecules with a small trans-influence are located in a trans position to each other and therefore are not replaced by a thiocheaver.
The laws of trans-influence were opened by I.I. Chernyaev, when studying the reactions of ligand substitution in flat-square platinum complexes (II. ). In the future, it was shown that the trans-influence of ligands is also manifested in complexes of other metals (Pt (IV), PD (II), CO (III), CR (III), RH (III), IR (III )) And another geometric structure. True, the rows of the trans-influence of ligands for different metals differ somewhat.
It should be noted that the trans influence is kinetic effect- The larger trans-influence possesses this ligand, with the greater speed another ligand is replaced with respect to it in the trans position.
Along with the kinetic effect of trans influence, in the middleXX century A.A. Greenberg and Yu.N. Cuccushkin Installed Dependence of Trans-Influence of LigandL. from the ligand located in the cis-position toL. . So, the study of the reaction rate of substitutionCl - ammonia in platinum complexes (Ii):
[PTCl 4] 2- + NH 3 \u003d [PTNH 3 Cl 3] - + Cl - K \u003d 0.42. 10 4 l / mol. from
[PTNH 3 Cl 3] - + NH 3 \u003d Cis- [PT (NH 3) 2 Cl 2] + Cl - K \u003d 1.14. 10 4 l / mol. from
trans- [PT (NH 3) 2 Cl 2] + NH 3 \u003d [PT (NH 3) 3 Cl] + + Cl - k \u003d 2.90. 10 4 l / mol. from
it showed that the presence in the cis-position to the substituted chloride ligand of one and two ammonia molecules leads to a sequential increase in the reaction rate. This kinetic effect was called cis-influence. Currently, both kinetic effects of the influence of the nature of ligands on the rate of reaction reactions of ligands (trans- and cis-influence) are combined in the general concept mutual influence of ligands.
The theoretical substantiation of the effect of the mutual influence of ligands is closely associated with the development of ideas about chemical bonds in complex compounds. In the 30sXX century A.A. Greenberg and B.V. Nekrasov trans-influenced as part of the polarization model:
1. Trans-influence is typical for complexes, the central ion of metal of which has a large polarizability.
2. The trans-activity of ligands is determined by the energy of the mutual polarization of the ligand and the metal ion. For this metal ion, the trans-influence of the ligand is determined by its polarizability and the distance from the central ion.
The polarization model is consistent with experimental data for complexes with simple anionic ligands, such as halide ions.
In 1943 A.A. Greenberg put forward the assumption that the trans-activity of ligands is associated with their recovery properties. The displacement of the electron density from the trans-active ligand to the metal reduces the effective charge of the metal ion, which leads to the weakening of the chemical bond with the trans-located ligand.
The development of submissions about trans-influence is associated with the large trans-activity of ligands on the basis of unsaturated organic molecules like ethylene in [Pt (C 2 H 4) Cl 3 ] -. According to Chatta and Orders (Fig. 46), this is due topithe dative interaction of such ligands with metal and the associative mechanism of reactions of reactions of trans-located ligands. Coordination to the metal attacking metal ionZ. leads to the formation of five-coordinate trigonal biysuit-donal intermediates, followed by the rapid cleavage of the leaving ligand X. The formation of such an intermediate contributes topifatal Ligand Metal Interconnection of LigandY. reducing the electronic density of the metal and reduces the activation energy of the transition state followed by the rapid substitution of the Ligand X.
As well as p. Acceptor (C 2 H 4, CN -, CO ...) ligands forming a dative ligand metal chemical bond, high trans-influence possesss. Donor Ligands:H -, CH 3 -, C 2 H 5 - ... the trans-influence of such ligands is determined by the donor-acceptor interaction of the ligand X with metal, lowering its electrical density and weakening the metal connection with the outgoing ligandY.
Thus, the position of ligands in a series of trans-activity is determined by the joint action of the sigma Donor I. pi properties of ligands - sigmadonor I. pithe acceptor properties of the ligand enhance its trans-influence, whilepi Donor - weaken. Which of these components of the ligand metal of interaction prevails in the trans-influence judged on the basis of the quantum-chemical calculations of the electronic structure of the transition state of the reaction.