Paul biochemistry. Oxidation of fatty acids changes the properties of cell membranes
Among the primary cell damage mechanisms for oxidative stress, the oxidation of fat-acid residues in membranes phospholipids is leading. This reduces their hydrophobicity and disrupts the stability of the membrane, changes the operation of membrane-bound enzymes, increases the permeability of membranes for ions.
The reactions of the interaction of free radicals with fatty acids were widely known in connection with their relevance in the food industry. The appearance of unpleasant smell and forki products - this is a manifestation lipid peroxidation(FLOOR).
The main substrate for free-radical reactions are double bonds of polyunsaturated fatty acids. IN cell membranes Polyunsaturated fatty acids are in the composition of phospholipids and glycolipids. Also, a large amount of phospholipids with polyunsaturated fatty acids are localized in sheath Lipoprotein High, low and very low density, which is in the pathogenesis of atherosclerosis.
As a result of free radical oxidation of fatty acids, hydropercycles are formed and dien conjugates (primary products), which are very unstable. With the participation of metals of valence variable, they are rapidly metabolized into secondary ( aldehydes and dialdehydes) and tertiary ( schiffov base) Lipid peroxidation products.
The peroxidation oxidation of lipids includes several stages:
- Initiation.
- Development.
- Branching.
- Crane chain.
At the time of initiation , for example, hydroxylthe radical is attacked by a methylene group located between double bonds, and a hydrogen atom is knocked out, restoring the hydroxyl radical to water. Next, in fatty acid, the double bond is rearranged, the displacement of the radical group and the interaction of it with oxygen. As a result, it is formed lipoperoxyl radical.
Further interaction of the obtained lipoperoxyl radical with adjacent fatty acids leads to its neutralization and the emergence of new lipoprotection radicals, i.e. to the development of a linear chain reaction with the appearance of new oxidized fatty acids.
Development of lipid peroxidation reactions
In addition to linear development, the reaction branch may occur by receiving hydroelectron peroxide from any metals or when exposed to radiation.

Branch and breaking of lipid peroxidation reactions
The breakage of the chain reaction occurs in the interaction of radicals with each other or in reaction with different antioxidants, for example, vitamin E, which gives the electrons, turning into a rather stable oxidized form.
Lipid peroxidation products
Primary food floor are hydropercy fatty acidsThey are subjected to further decay with the formation of secondary food products, various alcohols, ketones, aldehydes and dialdehydes, epoxides and other compounds.
Most reactive out secondary Floor products is mail Dialdehyde (MDA), which is capable of forming covalent bonds with NH 2-groups of proteins and other molecules with the formation of spoff bases.

Malone Dialdehyde Education Reaction Scheme
The role of Malon Dialdehyde
(MDA), formed when lipid peroxidation peroxide, is able to react with ε-NH 2 -Groups lizina or N-terminal protein amino acids, with NH 2 -Groups phospholipid and glycozamines. MDA forms bridges inside molecules and between them with the formation of spoff bases.
Active forms of oxygen (free radicals)
In the body as a result oxidative - recoveful reactions are constantly being a permanent oxygen forms (AFC) with one-electron reduction of oxygen (the molecule has an unpaired electron on a molecular or external atomic orbit).
Sources of AFK:
1) Chain of tissue respiration (leakage of electrons with reduced ubiquinone koqh 2 per oxygen);
2) reactions catalyzed by oxidases, hematoproteins, cytochrome P 450;
3) the oxidation reactions in leukocytes, macrophages and peroxyms;
4) radioliz of water;
5) under the influence of xenobiotics, pesticides;
6) the reaction of spontaneous (non-enzymatic) oxidation of a number of substances.
Superoxide Anion. - It is one of the most widespread free radicals in the body:
It is formed in the cells of pathogenic bacteria and is a damaging factor for the membranes of the cells of the parenchymal organs of the human body. For leukocytes and macrophages, the superoxide anion is a factor of bactericidality, with which the cells inactivate pathogenic microorganisms.
Another way of formation of free radicals is the interaction of oxygen with valence variable metals. At the same time it is formed peroxidaDical:
Fe 2+ + O 2 + H + → Fe 3+ + HO 2
O 2 - + H + → HO 2
The interaction of a superoxidanion with a peroxide radical (1) or one-electron restoration of superoxide anion (2) in the aqueous medium leads to education hydrogen peroxide
O 2 - + but 2 + H + → H 2 O 2 + O 2 (1)
O 2 - + E - + 2N + → H 2 O 2 (2)
Hydroxyl radical ON. It is formed in the interaction of hydrogen peroxide with superoxide anion (1) or with metals (2):
H 2 O 2 + O 2 - → He + it - + O 2 (1)
H 2 O 2 + FE 2+ → It + it - + Fe 3+ (2)
Oxygen radicals have a high reactivity and easily enter into chemical reactions with organic molecules to purchase a missing electron. Oxygen radicals have an impact on various structural components of cells: DNA (damage to nitrogen bases); proteins (oxidation of amino acid residues, the formation of covalent "crosslocks"); lipids; Membrane structures.
The active forms of oxygen can eliminate electrons from many compounds, turning them into new free radicals, thereby initiate chain oxidative reactions. If unsaturated oxidic acids of plasma membranes enter into the reaction with AFC, they are talking about lipid peroxidation.
The reactions floor are free radical and constantly flow in the body, as well as the reaction of EFC education. Normally, they are supported at a certain level and perform a number of functions:
· Apoptosis (Programmed cell death);
· Regulate the structure of cell membranes and thereby ensure the functioning of ion channels, receptors, enzyme systems;
· Provide exemption from the arachidonic acid membrane, from which bioregulators (prostaglandins, thromboxanes, leukotrienes) are synthesized;
· The floor can act as a secondary messenger, participating in the transformation of signals from the external and internal environment of the body, providing their intracellular transmission;
· AFC is involved in cellular immunity and phagocytosis.
Mechanism floor :
1) initiation.
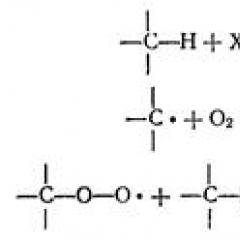
It initiates the reaction most often hydroxyl radical, taking hydrogen from CH 2 - groups of unsaturated fatty acid L, which leads to the formation of a lipid radical L ·:
L + Oh → L ·2) the development of the chain.
The development of the chain occurs when the oxygen is connected, as a result of which the peroxide radical loo is formed · or Lipid Lipid peroxide (lipid hydropercy)
L · + o 2 → Loo ·
Lo · + LH → Looh + LR · ·
3) circuit breaking.
The development of the chain may stop in the interaction of free radicals among themselves or when interacting with different antioxidants (vitamin E), which are electrons donors:
Loo · ∙ + L · → Looh + LH
L ∙ · + WIT E → LH + WIT E · ∙
WIT E · + L · → LH + WIT E Oxid
As a result, the floor is converted by conventional lipids in primary Food Products (Lipid Hydroperies). This leads to the appearance of areas in the membranes ("holes"), through which the contents of both the cells themselves and their organelles are coming out.
Primary foods floor are destroyed with education secondary products floor: aldehydes, ketones, malonic dialdehyde, diene conjugates. The accumulation of Malone Dialdehyde (MDA) is explained by intoxication syndrome accompanying many diseases of the internal organs. Responding to SH- and CH 3-groups of proteins, MDA suppresses the activity of cytochrome-oxidases (inhibited by tissue breathing) and hydroxylase. Hmm also causes the accelerated development of atherosclerosis.
When interacting by MDA with the amino groups of phospholipids are formed end products floor - Schiffs foundation. An example of these compounds is the pigment of lipofuscin appearing on the shell of the eye on the skin with age. Lipofuscin is a mixture of lipids and proteins associated with transverse covalent bonds and tested as a result of interaction with chemically active groups of floors. This pigment is phagocytic, but not hydrolyzed by enzymes lysosomes, accumulates in cells, violating their function.
Negative effects of activation gender :
· Damage to lipid bilayer membranes, as a result of which water penetrates into the cells, sodium ions, calcium, which leads to the swelling of cells, organelle and their destruction.
· Premature aging of cells and the body as a whole.
· Interaction of high-formative food products with amino groups of proteins with the formation of spoff bases.
· Change the flow rate (viscosity) membranes, as a result of which the transport function of membranes (functioning of ion channels) is broken.
· Violation of the activity of membrane-bound enzymes, receptors.
Activation floor Characteristic for many diseases and pathological conditions:
· Atherosclerosis and other cardiovascular disease;
· TsNS lesions (Parkinson's disease, Alzheimer);
· Inflammatory processes of any genesis;
· Muscle dystrophy (Dungal disease);
· oncological diseases;
· Radiation lesions;
· Bronchildren's pathology.
The lipid peroxidation (auto-oxidation) of lipids during contact with oxygen does not only cause dissenting food (downtown), but also causes damage to tissues in vivo, contributing to the development of tumor diseases. The damaging effect is initiated by free radicals arising during the formation of fatty acid peroxides containing double bonds alternating with methylene bridges (such an alternation is available in natural polyunsaturated fatty acids) (Fig. 15.28). Lipid peroxidation is a chain reaction that provides extended reproduction of free radicals that initiate further distribution of peroxidation. The whole process can be represented as follows.
1) initiation: education R from the predecessor
2) Development of the reaction:
3) Termination (cessation of reaction):

Since ROOH hydraulic acts as a predecessor in the initiation process, lipid peroxidation is a branched chain reaction, potentially capable of causing significant

Fig. 15.27. Dolichol (-Spirt).

Fig. 15.28. Lipid peroxidation. The reaction is initiated by light or metal ions. Malonic dialdehyde, which is formed only from fatty acids with three and more double bonds, is used as an indicator of lipid peroxidation, together with the ethane, resulting from the cleavage of an end two-carbon fragment of-sliced \u200b\u200bacids, and pentane, formed when the terminal five-carbon fragment is selected. .
damage. To regulate the process of peroxidation oxidation of fats and man, and nature use antioxidants. Propillatals, bottled hydroxyanisole and bottled hydroxitoluluole are added to the foods for this purpose. Natural antioxidants include fat-soluble vitamin E (tocopherol), as well as water-soluble urates and vitamin C. -Karotin is an antioxidant only at low values \u200b\u200bof antioxidants disintegrated into two classes: 1) preventive antioxidants that reduce the speed of initiation of the chain reaction, and 2) quenching (interrupting Chain) Antioxidants that prevent the development of a chain reaction. The first is the first catalase and other peroxidases that destroy ROOH, and agents forming chelated complexes with Metals -DTP A (Diethylene ParantateAtate) and EDTA (ethylenediaminetetraacetate). Phenols or aromatic amines are often performed as an interrupting chain of antioxidants. Under conditions in vivo, the main interrupting chain antioxidants are superoxiddismutase (see p. 126), which in the aqueous phase captures superoxide free radicals as well as vitamin E, capturing free ROO radicals in the lipid phase, and possibly uric acid.
The peroxidation oxidation in vivo is also catalyzed by hem compounds and lipoxganasamn, bent in platelets, leukocytes, etc.

Fig. 15.29. a-tocopherol.
Vitamin E (a-tocopherol)
There are several natural tocopherols. All of them are 6-hydroxychromanes or tocolas with isoprene substituents (Fig. 15.29). A-tocopherol is most widespread and has the greatest biological activity as vitamin.
Vitamin E performs at least two metabolic functions. Firstly, it serves as the most potent natural fatty-soluble antioxidant and, secondly, performs specific, although not to the end understandable, the role in the metabolism of Selena.
Vitamin E, apparently, is the first echelon of the protection of cellular and subcellular membrane phospholipids from peroxidation oxidation. The phospholipids of mitochondria, the endoplasmic reticulum and plasma membranes have specific affinity for A-tocopherol, therefore vitamin, apparently, concentrates in the composition of these membranes. Tocopherols act as antioxidants, interrupting oxidation chains due to their ability to carry phenolic hydrogen to peroxide radical (Fig. 15.30). The phenoxyradecal is a resonant-stabilized and relatively nonreactive structure, with the exception of its interaction with other peroxide radicals. Thus, the a-tocopherol is almost not involved in the process of chain oxidation reaction; When oxidizing the chromium ring and the side chain of A-tocopherol, a product is formed that is not a free radical (Fig. 15.31). This product forms a conjugate with glucuronic acid and excursed with biliary. The antioxidant effect of A-tocopherol is preserved at high oxygen concentrations, so it is not surprising that
Fig. 15.30. The antioxidant effect of tocopherols in relation to peroxidant radicals

Fig. 15.31. A-tocopherol oxidation product. The numbering of atoms allows you to compare their position in the product and the initial connection.
vitamin accumulates in lipid-rich areas in contact with the medium, where high partial oxygen pressure is maintained, - in the erythrocyte membranes and respiratory cells.
However, even in the presence of an adequate amount of vitamin E, there is a certain number of peroxides. The second echelon of protection of the membrane from the destructive action of peroxide (see p. 204) is glutalone-peroxidase, which includes selenium. Thus, the effect of vitamin E and selenium consists, apparently, in the protection of cellular and subcellular components from damage to the peroxides, ensuring the integrity of the organelle and thereby preventing the development of pathological conditions under the action of physical, chemical or other stress factors.
Among the products of this process - Malondiadehyde and 4-hydroxinenal.
The reactions of biological oxidation are accompanied by the formation of free radicals, particles having an unpaired electron in an external orbit. This causes the high chemical activity of these radicals. For example, they react with unsaturated fatty acids of membranes, violating their structure. Antioxidants prevent free radical oxidation.
Through the stage of peroxidant derivatives of unsaturated fatty acids, the biosynthesis of prostaglandins and leukotrienes is carried out, and thromboxanes that have a powerful influence on the adhesive-aggregation properties of uniform elements of blood and microcirculation are hydraulic. The formation of cholesterol hydroperosis is one of the links in the synthesis of some steroid hormones, in particular, progesterone.
Literature
- Vladimirov Yu.A., Archakov A.I. Lipid peroxidation in biological membranes. - M.: Science, 1972. - 252 p.
- Baraba V. A., Orel V.E., Karnukh I.M. Peroxidation and radiation. - K.: Nukova Dumka, 1991.
- K. V. bucket - Free radical oxidation
Notes
Wikimedia Foundation. 2010.
Watch what is "lipid peroxidation" in other dictionaries:
lipid peroxidation - The process of the interaction of lipids (their unsaturated areas), which are included in cell membranes with oxidizing agents (Anion O2, radical, etc.), which are formed under the action of ionizing irradiation and in the metabolic processes of some substances; ... Technical translator directory
Lipid peroxidation peroxidation of lipids. The process of interaction of lipids (their unsaturated areas), which are included in cell membranes, with oxidizing agents (anion O2, radical, but also), which are subtracted under the action of ionizing ... ... Molecular biology and genetics. Dictionary.
A complex multistage chain process of oxygenation oxygen by lipid substrates, mainly polyunsaturated fatty acids, including the stages of the interaction of lipids with free radical compounds and the formation of free lipid radicals ... Medical encyclopedia
Mechanism floor. Lipid peroxidation (floor) oxidative lipid degradation occurring mainly under the action of free radicals. One of the main effects of irradiation. One of the products of this process Malondiadehyde. Literature Yu ... Wikipedia
With diabetes mellitus, the body develops a lack of vitamins and minerals. This is due to three reasons: limiting the diet, impairment of substances and a decrease in the assimilation of beneficial substances. In turn, the deficit of vitamins and ... ... Wikipedia
- (Dibunolum) (see also tocopherol acetate). 2,6 di Troket Butyl 4 methylphenol. Synonyms: butyloxitoluene, ionol. White or white with a slightly yellowish tint of crystalline powder. Practically insoluble in water, easily soluble in alcohol. ... ...
Dibunol (Dibunolum) (see also tocopherol acetate). 2,6 di Troket Butyl 4 methylphenol. Synonyms: butyloxitoluene, ionol. White or white with a slightly yellowish tint of crystalline powder. Practically insoluble in water, easily soluble in alcohol ... Dictionary of medical preparations
I Fatty acid carboxylic acids; In the body of animals and in plants are free and incoming fatty acids in the lipid perform energy and plastic functions. J. K. The composition of phospholipids participate in the construction of biological ... ... Medical encyclopedia
Atoms or groups of chemically coupled atoms with free valences, i.e. unpaired (uncompensated) electrons on the outer (valence) orbital. The presence of unpaired electrons determines the high chemical reaction ... ... Medical encyclopedia
Active substance \u003e\u003e Amino acids for parenteral nutrition + other preparations [Fat emulsions for parenteral nutrition + dextrose + mineral salts] (Aminoacids for Parenteral Nutrition + Other Medicines)