Primary alcohols are formed at. Alcohol
Definition
Alcohol - Compounds containing one or more hydroxyl groups -One associated with a hydrocarbon radical.
Depending on the number of hydroxyl groups, alcohols are divided into one- (CH 3 OH - methanol, 2 H 5 OH - ethanol), two- (CH 2 (OH) -CH 2 -OH - ethylene glycol) and Trehatomic (CH 2 (OH) -CH (OH) -CH 2 -OH - glycerin). Depending on whether the carbon atom is a hydroxyl group, the primary (R-CH 2 -OH) is distinguished, secondary (R 2 CH-OH) and tertiary alcohols (R 3 C-OH). In the title of alcohols there is a suffix - ol.
Monatomatic alcohols
The general formula of the homologous series of limit monohydric alcohols C n h 2 n +1 OH.
Isomeria
For extreme monohydric alcohols, the isomerism of the carbon skeleton is characteristic of the isomerization of the carbon skeleton (starting from butanol), as well as the isomerization of the position of the hydroxyl group (starting with propanol) and interclative isomerism with simple ethers.
CH 3 -CH 2 -CH 2 -CH 2 -One (Butanol - 1)
CH 3 -CH (CH 3) - CH 2 -On (2-methylpropanol - 1)
CH 3 -CH (OH) -CH 2 -CH 3 (Butanol - 2)
CH 3 -CH 2 -O-CH 2 -CH 3 (diethyl ether)
Physical properties
Lower alcohols (up to 15) - liquids, higher - solids. Methanol and ethanol are mixed with water in any ratios. With the increasing molecular weight, the solubility of alcohols in ODE falls. Alcohols have high boiling and melting temperatures due to the formation of hydrogen bonds.
Getting alcohols
Obtaining alcohols is possible using biotechnological (fermentation) of wood or sugar method.
Laboratory methods for obtaining alcohols include:
- hydration of alkenes (the reaction proceeds when heated and in the presence of concentrated sulfuric acid)
CH 2 \u003d CH 2 + H 2 Oh → CH 3
- hydrolysis of alkyl halides under the action of aqueous solutions alkalis
CH 3 BR + NaOH → CH 3 OH + NABR
CH 3 BR + N 2 O → CH 3 OH + HBr
- Restoration of carbonyl compounds
CH 3 -CH-O + 2 [H] → CH 3 - CH 2 -OH
Chemical properties
1. Reaction leaking with a bond of communication ON:
- Acid properties of alcohols are very weak. Alcohols react with alkaline metals
2C 2 H 5 OH + 2K → 2C 2 H 5 OK + H 2
but do not react with alkalis. In the presence of water, alcoholates are completely hydrolyzed:
C 2 H 5 OK + H 2 O → C 2 H 5 OH + KOH
This means that alcohols are weaker acids than water
- the formation of esters under the action of mineral and organic acids:
CH 3 -CO-OH + H-OCH 3 ↔ CH 3 COOCH 3 + H 2 O
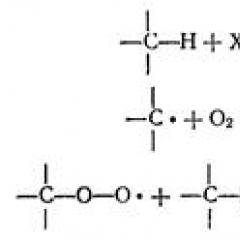
- oxidation of alcohols under the action of dichromate or permanganate potassium to carbonyl compounds. Primary alcohols are oxidized in aldehydes, which, in turn, can oxidize in carboxylic acids.
R-CH 2 -OH + [O] → R-CH \u003d O + [O] → R-COOH
Secondary alcohols are oxidized in ketones:
R-CH (OH) -R '+ [O] → R-C (R') \u003d O
Tertiary alcohols are more resistant to oxidation.
2. Reaction with the breakdown of C-O.
- intramolecular dehydration with the formation of alkenes (occurs at strong heating of alcohols with water-based substances (concentrated sulfuric acid)):
CH 3 -CH 2 -CH 2 -OH → CH 3 -CH \u003d CH 2 + H 2 O
- Intermolecular dehydration of alcohols with the formation of ethers (occurs at low heating of alcohols with water-based substances (concentrated sulfuric acid)):
2C 2 H 5 Oh → C 2 H 5 -O-C 2 H 5 + H 2 O
- Weak basic properties of alcohols are manifested in reversible reactions with halogen hydrogen:
C 2 H 5 OH + HBr → C 2 H 5 BR + H 2 O
Examples of solving problems
Example 1.
| The task | Determine the molar mass and the structure of the alcohol, if it is known that with the interaction of 7.4 g of this alcohol with metallic sodium, 1.12 liters of gas is distinguished (N.U.), and when oxidizing copper oxide (II), a compound is formed, which gives the reaction " Silver mirror. |
| Decision | We will make the equation of the reactions of alcohol ROH C: a) sodium; b) Cuo oxidizer: From equation (a) by the method of relations to determine the molar mass of the unknown alcohol: 7,4/2h. = 1,12/22,4, h. = M.(ROH) \u003d 74 g / mol. Such a molar mass has alcohols with 4 H 10 O., according to the condition of the problem [Equation (b)], these can be primary alcohols - butanol-1 CH 3 CH 2 CH 2 CH 2 OR or 2-METILPROPANOL-1 (CH 3) 2 SNSH 2 he. |
| Answer | M (with 4 H 10 o) \u003d 74 g / mol, it is butanol-1 or 2-methylpropanol-1 |
Example 2.
| The task | What volume (in l) oxygen (N.U.) will be required for complete combustion of 31.25 ml of ethyl alcohol (density of 0.8 g / ml) and how many grams of the sediment turn out when the reaction products are passed through lime water? |
| Decision | We will find the mass of ethanol: m. = × V. \u003d 0.8 × 31.25 \u003d 25 The amount of substance corresponding to this mass: (C 2 H 5) \u003d m / m \u003d 25/46 \u003d 0.543 mol. We write ethanol burning response equation: The volume of oxygen consumed during the combustion of ethanol: V. (O 2) \u003d 25 × 3 × 22.4 / 46 \u003d 36.5 liters. According to coefficients in the reaction equation: (O 2) \u003d 3 (from 2 H 5) \u003d 1.63 mol, (CO 2) \u003d 2 (C 2 H 5) \u003d 1.09 mol. |
The total formula of the homologous series of limit monohydric alcohols - C n h 2N + 1 OH. Depending on whether the carbon atom is a hydroxyl group, the primary alcohols are distinguished (RCh 2 -OH), secondary (R 2 CH-OH) and tertiary (R 3 C-OH). The simplest alcohols:
Primary:
CH 3 -one CH 3 -CH 2 -Hen CH 3 -CH 2 -CH 2 -H
methanol ethanol propanol-1
Secondary tertiary
propanol-2 Buiganol-2 2-methylpropanol-2
Isomeriasingoatomic alcohols are associated with the structure of a carbon skeleton (for example, butanol-2 and 2-methylpropanol-2) and with the position of the group it (propanol-1 and propanol-2).
Nomenclature.
The names of the alcohols form, adding the end-to the name of the hydrocarbon title with the longest carbon chain, including the hydroxyl group. The numbering of the circuit starts from that edge, closer to which the hydroxyl group is located. In addition, the substitution nomenclature is widespread, according to which the name of the alcohol is made from the appropriate hydrocarbon radical with the addition, the words "alcohol", for example: C 2 H 5 OH - ethyl alcohol.
Structure:
Alcohol molecules have an angular structure. The angle R-O-H in the methanol molecule is 108.5 0. An oxygen atom of the hydroxyl group is in SP 3-hybridization.
Getting. Properties
Getting.
1. The most general way to obtain alcohols, which has industrial importance, is hydration of alkenes. The reaction goes when alkenet passes with water pairs above the phosphate acid catalyst:
CH 2 \u003d CH 2 + H 2 O → CH 3 -CH 2 -On.
Ethyl alcohol is obtained from ethylene, isopropyl. The connection of water goes according to the rule of Markovnikov, so only ethyl alcohol can be obtained from primary alcohols for this reaction.
2. Another common method of obtaining alcohols is hydrolysis of alkyl halides under the action of aqueous solutions by alkalis:
R-BR + NaOH → R-OH + NaBr.
For this reaction, you can get primary, secondary and tertiary alcohols.
3. Restoration of carbonyl compounds. When restoring the aldehydes, primary alcohols are formed, when the ketone is restored - secondary:
R-CH \u003d O + H 2 → R-CH 2 -OH, (1)
R-CO-R "+ H 2 → R-CH (OH) -R." (2)
The reaction is carried out, passing a mixture of aldehyde or ketone vapor and hydrogen over a nickel catalyst.
4. The effect of Grignar reagents on carbonyl compounds.
5. Ethanol is obtained by alcohol fermentation glucose:
C 6 H 12 O 6 → 2C 2 H 5 OH + 2SO 2.
Chemical properties Alcohols are determined by the presence in their molecules of the hydroxyl group it. Communication C-O and O-N are very polar and capable of rupture. There are two basic types of alcohol reactions with the participation of the functional group -on:
1) the reaction with the break-up of the O-H: a) the interaction of alcohol with alkaline and alkaline earth metals to form alcoholates; b) the reactions of alcohols with organic and mineral acids with the formation of esters; c) oxidation of alcohols under the action of dichromate or potassium permanganate to carbonyl compounds. The rate of reactions in which the connection of ON is broken, decreases in a row: primary alcohols\u003e secondary\u003e tertiary.
2) reactions accompanied by a breakdown of C-O: a) catalytic dehydration with the formation of alkenes (intramolecular dehydration) or ethers (intermolecular dehydration): b) the replacement of the group -On halogen, for example, under the action of halogen breeds to form alkyl halides. The rate of reactions in which the connection of C - O is broken, decreases in a row: tertiary alcohols\u003e secondary\u003e primary. Alcohols are amphoteric compounds.
Reactions with a break-up O-N.
1. Acid properties of alcohols are very weak. Lower alcohols are rapidly reacting with alkaline metals:
2C 2 H 5 -On + 2K → 2C 2 H 5 -K + H 2, (3)
but do not react with alkalis. With an increase in the length of the hydrocarbon radical, the speed of this reaction slows down.
In the presence of traces of moisture of alcohol salts (alcohologists) decompose to the source alcohols:
C 2 H 5 OK + H 2 O → C 2 H 5 ON + KON.
This proves that alcohols are weaker acids than water.
2. Under action on alcohols of mineral and organic acids, sophisticated esters are formed. The formation of esters flows through the mechanism of nucleophilic joining-cleavage:
C 2N 5 ON + CH 3 coxy ![]() CH 3 coa 2 H 5 + H 2 o
CH 3 coa 2 H 5 + H 2 o
Ethyl acetate
C 2 H 5 OH + HONO 2 ![]() C 2 H 5 ONO 2 + H 2 O
C 2 H 5 ONO 2 + H 2 O
EthylNitrath
A distinctive feature of the first of these reactions is that the hydrogen atom is cleaved by alcohol, and the group it is from the acid. (Established experimentally by the "labeled atoms" method).
3. Alcohols are oxidized under the action of dichromate or potassium permanganate to carbonyl compounds. Primary alcohols are oxidized in aldehydes, which, in turn, can oxidize in carboxylic acids:
R-CH 2 -OH → R-CH \u003d O → R-COOH.
Secondary alcohols are oxidized in ketones:

Tertiary alcohols can only be oxidized with bonds gap.
Reactions with a break of communication C-O.
1) The dehydration reactions occur when heating alcohols with water-based substances. With strong heating, intramolecular dehydration occurs with the formation of alkenes:
H 2 SO 4, T\u003e 150 ° C
CH 3 -CH 2 -CH 2 -One → CH 3 -CH \u003d CH 2 + H 2 O.
With a weaker heating, intermolecular dehydration occurs with the formation of ethers:
H 2 SO 4, T< 150°С
2CH 3 -CH 2 -OH → C 2 H 5 -O-C 2 H 5 + H 2 O.
2) alcohols react react with halogen breeding acids (weak basic properties of alcohols appear here:
ROH + HCL ![]() RCL + H 2 O
RCL + H 2 O
Tertiary alcohols react quickly, secondary and primary - slowly.
Application.Alcohols are mainly used in the industry of organic synthesis. Ethanol is an important raw material industry. Used as a solvent, in medicine.
Methanol is used to produce formaldehyde, plastics based on acrylic acid, as a solvent for varnishes and paints.
Definition
Alcohol - Compounds containing one or more hydroxyl groups -One associated with a hydrocarbon radical.
The general formula of the homologous series of limit monohydric alcohols C n h 2 n +1 OH. In the title of alcohols there is a suffix - ol.
Depending on the number of hydroxyl groups, alcohols are divided into one- (CH 3 OH - methanol, C 2 H 5 OH - ethanol), two- (CH 2 (OH) -CH 2 -OH - ethylene glycol) and Trehatomic (CH 2 (OH ) -CH (OH) -CH 2 -OH - glycerin). Depending on whether the carbon atom is a hydroxyl group, the primary (R-CH 2 -OH) is distinguished, secondary (R 2 CH-OH) and tertiary alcohols (R 3 C-OH).
For extreme monohydric alcohols, the isomerism of the carbon skeleton is characteristic of the isomerization of the carbon skeleton (starting from butanol), as well as the isomerization of the position of the hydroxyl group (starting with propanol) and interclative isomerism with simple ethers.
CH 3 -CH 2 -CH 2 -CH 2 -One (Butanol - 1)
CH 3 -CH (CH 3) - CH 2 -On (2-methylpropanol - 1)
CH 3 -CH (OH) -CH 2 -CH 3 (Butanol - 2)
CH 3 -CH 2 -O-CH 2 -CH 3 (diethyl ether)
Chemical properties of alcohols
1. Reaction leaking with a bond of communication ON:
- Acid properties of alcohols are very weak. Alcohols react with alkaline metals
2C 2 H 5 OH + 2K → 2C 2 H 5 OK + H 2
but do not react with alkalis. In the presence of water, alcoholates are completely hydrolyzed:
C 2 H 5 OK + H 2 O → C 2 H 5 OH + KOH
This means that alcohols are weaker acids than water
- the formation of esters under the action of mineral and organic acids:
CH 3 -CO-OH + H-OCH 3 ↔ CH 3 COOCH 3 + H 2 O
- oxidation of alcohols under the action of dichromate or permanganate potassium to carbonyl compounds. Primary alcohols are oxidized in aldehydes, which, in turn, can oxidize in carboxylic acids.
R-CH 2 -OH + [O] → R-CH \u003d O + [O] → R-COOH
Secondary alcohols are oxidized in ketones:
R-CH (OH) -R '+ [O] → R-C (R') \u003d O
Tertiary alcohols are more resistant to oxidation.
2. Reaction with the breakdown of C-O.
- intramolecular dehydration with the formation of alkenes (occurs at strong heating of alcohols with water-based substances (concentrated sulfuric acid)):
CH 3 -CH 2 -CH 2 -OH → CH 3 -CH \u003d CH 2 + H 2 O
- Intermolecular dehydration of alcohols with the formation of ethers (occurs at low heating of alcohols with water-based substances (concentrated sulfuric acid)):
2C 2 H 5 Oh → C 2 H 5 -O-C 2 H 5 + H 2 O
- Weak basic properties of alcohols are manifested in reversible reactions with halogen hydrogen:
C 2 H 5 OH + HBr → C 2 H 5 BR + H 2 O
Physical properties of alcohol
Lower alcohols (up to 15) - liquids, higher - solids. Methanol and ethanol are mixed with water in any ratios. With the increasing molecular weight, the solubility of alcohols in ODE falls. Alcohols have high boiling and melting temperatures due to the formation of hydrogen bonds.
Getting alcohols
Obtaining alcohols is possible using biotechnological (fermentation) of wood or sugar method.
Laboratory methods for obtaining alcohols include:
- hydration of alkenes (the reaction proceeds when heated and in the presence of concentrated sulfuric acid)
CH 2 \u003d CH 2 + H 2 Oh → CH 3
- hydrolysis of alkyl halides under the action of aqueous solutions alkalis
CH 3 BR + NaOH → CH 3 OH + NABR
CH 3 BR + N 2 O → CH 3 OH + HBr
- Restoration of carbonyl compounds
CH 3 -CH-O + 2 [H] → CH 3 - CH 2 -OH
Examples of solving problems
Example 1.
| The task | Mass stakes of carbon, hydrogen and oxygen in the molecule of the limit monohydric alcohol 51.18, 13.04 and 31, 18%, respectively. Output alcohol formula. |
| Decision | Denote the number of elements included in the alcohol molecule indexes x, y, z. Then, the alcohol formula in general will look like - with x H y o z. We write the ratio: x: y: z \u003d ω (C) / AR (C): ω (H) / AR (H): Ω (O) / AR (O); x: y: z \u003d 51.18 / 12: 13,04 / 1: 31,18 / 16; x: y: z \u003d 4,208: 13.04: 1.949. We divide the resulting values \u200b\u200bfor the smallest, i.e. at 1.949. We get: x: Y: Z \u003d 2: 6: 1. Consequently, the alcohol formula is C 2 H 6 O 1. Or C 2 H 5 OH is ethanol. |
| Answer | The formula of the limit monohydric alcohol - C 2 H 5 Oh. |
1. Classification of hydroxyl derivative hydrocarbons.
2. Limit monatomatic alcohols (alkanolas).
3. Polymatomic alcohols.
4. Phenols.
5. Simple ethers.
Hydroxyl derivatives of hydrocarbons are compounds that are formed as a result of substitution in the hydrocarbon molecule of one or more hydrogen atoms into hydroxyl groups.
Hydroxyl derivative hydrocarbons with communication with (SP 3) -On are called alcohols. These are limit aliphatic and cyclic alcohols, for example, CH 3 is and,
unfoluble alcohols, for example CH 2 \u003d CH-CH 2 -On, aromatic alcohols -
Hydroxyl derivatives, comprising communication with (SP 2) -On, are called ENOLs R-CH \u003d CH-OH and Phenol
According to the number of hydroxyl groups contained in the molecule, alcohols and phenols may be one (one-group) -, two (two ON-groups) -, three- and polyatomic.
Finding in nature.In contrast to halogen derivatives of alcohol and phenol hydrocarbons, their derivatives are widely represented in the vegetable and animal world.
Higher alcohols are found in free form (for example, Cetyl alcohol with 16 H 33), as part of esters with higher fatty acids (spermacet, waxes). Unfoluble alcohols are an integral part of essential oils. Natural cyclic alcohols are menthol and cholesterol. Glycerin is part of natural vegetable and animal fats and oils.
Phenols and their ethers are part of the essential oils of many fragrant plants, for example, thyme, thyme, cumin, anise, etragon, dill, etc. Multiatomic phenols and their derivatives are fragrant substances of plants (for example, cloves, nutmeg), an integral part of the glycosides of plants, tanning substances of tea, coffee, etc.
1. Limit monatomatic alcohols (alkanols).
General formula with N H 2 n +1.
Nomenclature.Under the substitution nomenclature, the hydroxyl group in the title of alcohols is denoted by the suffix - ol.On the radical and functional nomenclature in the title indicate the radical and add - alcohol:From 2N 5 he - ethanol. or ethylalcohol,
CH 3 -CH 2 -CH 2 -One - propanol-1 or propyllittle alcohol.
Getting:
a) Hydrolysis Halogenels. Halogens in reactions with water or an aqueous alkali solution easily form alcohols (see "Halogen derivatives of hydrocarbons"):
C 2 H 5 VR + NAON (aqueous solution) → From 2N 5 he + Nav.
b) hydration of alkenes. The addition of water to alkens occurs in the presence of a catalyst (see "Alkenes"):
CH 2 \u003d CH 2 + H-it CH 3 -CH 2 -On.
c) hydrogenation of carbonyl compounds.
Catalytic hydrogenation of aldehydes and ketones leads to alcohol formation (see "Aldehydes and ketones"):
CH 3 -CH \u003d O + N 2 → CH 3 -CH 2 -H

Catalysts: Ni, Pt, PD.
d) the reaction of magniaorganic connections. The magniaorganic compounds to aldehydes and ketones are easily accounted for (see "Aldehydes and ketones"):
Primary alcohol is formed from methanal, from aldehydes - secondary alcohols, ketones - tertiary alcohols.
The feature of the reactions of this type is the reaction products - alcohols contain more carbon atoms compared to the initial carbonyl compounds.
e) carbon oxide hydrogenation (II). Depending on the nature of the catalyst and the reaction conditions, methanol or a mixture of different alcohols (synthol) are obtained: CO + 2H 2 → CH 3.
Catalysts: Zno, CO and others.
e) alcohol fermentation of carbohydrates. Glucose in the presence of yeast is subjected to fermentation with the formation of ethyl alcohol and carbon dioxide: from 6 H 12 O 6 → 2SH 3 -CH 2 -On + 2 - 2
Isomeria.For limit alcohols, structural isomeria is characteristic: isomerism of the carbon chain, the location of the hydroxyl group in the chain. According to the position of the hydroxyl group in the chain, the primary (R-CH 2 -On) is distinguished, secondary (R 2 CH-OH) and tertiary (R 3 s-OH) alcohols.
For alcohols, an inter-odd isomerism (metamery) is characterized, alcohols are an ether of ethers with a general formula R-O-R.
CH 3 -CH 2 - SNHe-CH 3 (see "Optical isomeria").
Structure.In alcohols, carbon and oxygen atoms are VSR 3 - hybridization. Alcohols contain two polar σ-bonds: C-O (SP 3 -SR 3-PREPARATION) and O-H (SP 3 -S-Apersion). The dipoles of these bonds are directed towards the oxygen atom, and the dipole moment of the O-H connection is higher than the relationship of C-O. Alcanolas are polar connections:
The association of alcohol molecules is carried out by the formation of intermolecular hydrogen bonds:

as a result of alcohols, compared with hydrocarbons and halogen derivatives of hydrocarbons, have higher boiling and melting temperatures. The formation of hydrogen bonds between alcohol and water molecules contributes to the dissolution of these compounds in water.
Chemical properties.
The chemical properties of alcohols are due to the presence in the molecule of polar bonds C-O and O-H and of the vulnerable electronic pairs on the oxygen atom.
a) acid properties
Alcohols are weak oh-n-acids. A row of acidity: RCO\u003e NON\u003e ROH.
In an aqueous solution, the acidity of alcohol themselves is reduced in the following direction: methanol\u003e primary\u003e secondary\u003e tertiary.
Acidic properties of alcohols are manifested in the formation of salts (alcoholate or alkoxides) when interacting with metals:
2C 2N 5 OH + 2NA → 2 C 2N 5 O - NA + + N 2
ethanol ethylate (ethoxide) sodium
In aqueous solutions, the salts are hydrolyzed to form alcohols and alkalis:
C 2 H 5 O - NA + + NON → C 2 H 5 ON + NAON
b) basic and nucleophilic properties
The main and nucleophilic properties of alcohols are due to the mean-free electronic pair on the oxygen atom.
The main properties are increasing in the next direction.
methanol.< первичные < вторичные < третичные спирты и проявляются в образовании оксониевых солей: С 2 Н 5 ОН + Н + → С 2 Н 5 ОН 2 + . Образование оксониевых солей играет важную роль в реакциях нуклеофильного замещения и отщепления.
Thus, alcohols are amphoteric compounds.
Weak nucleophilic properties of alcohols and alcoholites are manifested in reactions
Alkylation - interactions with alcohols and alcoholates with the formation of ethers (Williamson reaction, flows when heated): CH 3 B.r. + FROM 2 N. 5 ABOUTNA → C 2N 5 AUD 3 + NABR
methylbromide ethylate sodium methoxyethane,
Acylation - interactions with carboxylic acids and their derivatives with the formation of esters (esterification reaction, proceeds in the presence of a catalyst):
CH 3 s IS HE + FROM 2 N. 5 ABOUTH ↔ CH 3 coa 2 H 5 + non
acetic acid ethanol ethyl acetate,
With carbonyl compounds - the formation of semi-acetals and acetals:
ethanal Methanol 1-methoxyethanol 1,2-dimethoxyethanol.
Alcoholates compared with alcohols are stronger bases and nucleophiles.
c) reactions of replacement of the hydroxyl group (nucleophilic substitution -S N. )
Often in these reactions, the group is modified with mineral acids or Lewis acids (the formation of oxonium salts of Ryon 2 +). The modified hydroxyl group is easily replaced by a halogen atom, an amino and alkoxy group and other groups. The reaction activity of alcohols in these reactions increases in the following direction: primary< вторичные < третичные.
Examples of reactions. Replace the hydroxyl group on a halogen atom:
R- He +.SO. Cl. 2 → R-CL + NCL + SO 2
R- He +.R N.al 5 → R-NAL + Nnal + Ronal 3
R- He +. N- N.al→ R-NAL + Non.
The reaction activity of halogen breeds is increased in the direction of HCL< НBr <НJ. Однако иодоводород практически не используют в реакциях этого типа, поскольку он легко восстанавливает спирты до углеводородов.
Replacement of the hydroxyl group on the amino and alkoxy group:
R- He +.N. - N.N. 2 → R- NN 2 + Non.
R- He +. RosN → R-O-R + Nonsense
Interaction with mineral acids with the formation of esters:
R- He +.N. -ABOUTN.ABOUT 2 → R-ONO 2 + Non.
alkylnitrath
R- He +.N. -ABOUTS.ABOUT 3 → R-OS 3 + Non.
alkyl sulfate
The reactions of nucleophilic substitution proceed along the monomolecular (S n 1) or bimolecular (S n 2) mechanism.
d) the reaction of the cleavage of the hydroxyl group (E-type, the dehydration of alcohols)
Water cleaned occurs when heated in the presence of a catalyst - sulfur or phosphoric acid, zinc oxide or aluminum. The dehydration of alcohols with the formation of alkenes proceeds in accordance with the Rule Zaitseva: the hydroxyl group is cleaved from an α-carbon atom, hydrogen - from a less hydrogenated β-atom of alcohol carbon:
1-butanol 2-butene
The reaction activity of alcohols is increased in the following direction: primary< вторичные < третичные.
The reaction of the cleavage flows on monomolecular (E1) or bimolecular (E2) mechanism.
e) oxidation of alcohols
In oxidation reactions, primary alcohols are more active, tertiary alcohols in similar conditions are not oxidized. Oxidifiers: Potassium permanganate or potassium bichromate in an acidic environment. Primary alcohols are oxidized with the formation of aldehydes and further - carboxylic acids, secondary alcohols - ketones:
R-OH + [O] → R-CH \u003d Oh → R-coxy
R 2 CH-OH + [O] → R 2 C \u003d O
Primary and secondary alcohols can be converted to carbonyl compounds during dehydrogenation. Reactions occur at 400-500 0 s in the presence of catalyst - Cu / Ag:

These are derivatives of hydrocarbons in which one hydrogen atom is substituted on the hydroxy group. General formula of alcohols - CNH. 2 N. +1 Oh..
Classification of monatomic alcohols.
Depending on the position where IS HE-Group, distinguish:
Primary alcohols:
Secondary alcohols:

Tertiary alcohols:
 .
.
Isomerios of monatomic alcohols.
For simultan alcohol Characteristic isomerism of the carbon skeleton and isomerism of the position of the hydroxy group.
Physical properties of monohydric alcohols.
The reaction goes according to the rule of Markovnikov, so only singer alcohol can be obtained from primary alkenes.
2. Hydrolysis of alkyl halides when exposed to aqueous alkalis solutions:
If the heating is weak, then intramolecular dehydration occurs, as a result of which ethers are formed:
B) alcohols can react with halogen conditions, with tertiary alcohols react very quickly, and primary and secondary - slowly:

Application of monatomic alcohols.
Alcoholuse mainly in industrial organic synthesis, in the food industry, in medicine and pharmacy.