Sections of physical chemistry. Physical and colloidal chemistry Physicochemistry
PHYSICAL CHEMISTRY
§ 1. The subject of physical chemistry. Its meaning
The relationship of chemical and physical phenomena studies physical chemistry. This branch of chemistry is the boundary between chemistry and physics. Using the theoretical and experimental methods of both sciences, as well as its own methods, physical chemistry is engaged in a multifaceted study of chemical reactions and the physical processes accompanying them. Since, however, even a multifaceted study is never complete and does not cover the phenomenon in an exhaustive way, the laws and laws of physical chemistry, like those of other natural sciences, always simplify the phenomenon and do not fully reflect it.
The rapid development and growing importance of physical chemistry are associated with its boundary position between physics and chemistry. The main general task of physical chemistry is the prediction of the time course of the process and the final result (equilibrium state) under various conditions based on data on the structure and properties of the substances that make up the system under study.
§ 2. Brief outline of the history of the development of physical chemistry
The term "physical chemistry" and the definition of this science were first given by M.V. Lomonosov, who in 1752-1754. read a course in physical chemistry to the students of the Academy of Sciences and left the manuscript of this course "Introduction to True Physical Chemistry" (1752). Lomonosov carried out many studies, the topics of which correspond to the "Plan for the course of physical chemistry" compiled by him (1752) and the program of experimental work "Experience in Physical Chemistry" (1754). Under his leadership, a student workshop in physical chemistry was also held.
Lomonosov gave the following definition of physical chemistry: "Physical chemistry is a science that explains, on the basis of the provisions and experiments of physics, what happens in mixed bodies during chemical operations." This definition is close to modern.
For the development of physical chemistry, the discovery of two laws of thermodynamics in the middle of the 19th century (S. Carnot, Yu.R. Mayer, G. Helmholtz, D.P. Joule, R. Clausius, W. Thomson) was of great importance.
The number and variety of research, lying in the field that borders between physics and chemistry, constantly increased in the 19th century. The thermodynamic theory of chemical equilibrium was developed (K.M. Guldberg, P. Waage, D.W. Gibbs). The studies of L.F. Wilhelmi laid the foundation for the study of the rates of chemical reactions (chemical kinetics). The transfer of electricity in solutions was studied (I.V. Gittorf, F.V.G. Kolrausch), the laws of equilibrium of solutions with steam were studied (D.P. Konovalov) and the theory of solutions was developed (D.I. Mendeleev).
The recognition of physical chemistry as an independent science and academic discipline was expressed in the establishment at the University of Leipzig (Germany) in 1887 of the first department of physical chemistry headed by W. Ostwald and in the foundation of the first scientific journal on physical chemistry there. At the end of the 19th century, the University of Leipzig was the center for the development of physical chemistry, and the leading physical chemists were W. Ostwald, J. H. Van't Hoff, S. Arrhenius and W. Nernst. By this time, three main sections of physical chemistry were defined - chemical thermodynamics, chemical kinetics and electrochemistry.
The most important areas of science, the development of which is a necessary condition for technical progress, include the study of chemical processes; physical chemistry plays a leading role in the development of this problem.
§ 3. Sections of physical chemistry. Research methods
Chemical thermodynamics. In this section, on the basis of the laws of general thermodynamics, the laws of chemical equilibrium and the doctrine of phase equilibria are expounded.
The doctrine of solutions aims to explain and predict the properties of solutions (homogeneous mixtures of several substances) on the basis of the properties of the substances that make up the solution.
The doctrine of surface phenomena. Various properties of surface layers of solids and liquids (interfaces between phases) are studied; one of the main studied phenomena in the surface layers is adsorption(accumulation of matter in the surface layer).
In systems where the interfaces between liquid, solid, and gaseous phases are highly developed (emulsions, mists, smokes, etc.), the properties of the surface layers become of primary importance and determine many of the unique properties of the entire system as a whole. Such dispersed (microheterogeneous) systems are being studied colloid chemistry, which is a major independent branch of physical chemistry.
The above list of the main sections of physical chemistry does not cover some areas and smaller sections of this science, which can be considered as parts of larger sections or as independent sections of physical chemistry. It should be emphasized once again the close interrelationship between the various branches of physical chemistry. In the study of any phenomenon, one has to use an arsenal of ideas, theories and methods for studying many branches of chemistry (and often other sciences). Only with an initial acquaintance with physical chemistry is it possible for educational purposes to distribute the material into the indicated sections.
Methods of physical and chemical research. The basic methods of physical chemistry are naturally the methods of physics and chemistry. This is, first of all, an experimental method - the study of the dependence of the properties of substances on external conditions, the experimental study of the laws of the flow of various processes and the laws of chemical equilibrium.
The theoretical understanding of experimental data and the creation of a coherent system of knowledge is based on the methods of theoretical physics.
The thermodynamic method, which is one of them, makes it possible to quantitatively relate various properties of a substance (“macroscopic” properties) and calculate some of these properties based on the experimental values of other properties.
CHAPTER I
THE FIRST LAW OF THERMODYNAMICS
§ 1. Energy. The law of conservation and transformation of energy
An integral property (attribute) of matter is movement; it is indestructible, like matter itself. The motion of matter manifests itself in different forms, which can pass one into another. The measure of motion of matter is energy. Quantitatively, energy is expressed in a certain way through the parameters characteristic of each specific form of movement, and in units specific to this form.
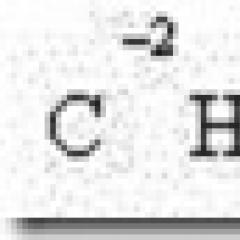
In the SI system of units, the unit of energy (heat and work) is the joule ( J), equal to the work of force in 1 H on the way to 1 m. 1 J = 1 Nm.
The widely used unit of energy (heat), the calorie, is currently an off-system unit that is allowed for use. The currently used calorie, by definition, equates to a certain number of joules: 1 feces equals 4.1868 joules. This unit is used in heat engineering and can be called thermal calorie. In chemical thermodynamics, a slightly different unit is used, equated to 4.1840 joules and called thermochemical calorie. The expediency of its application is connected with the convenience of using the extensive experimental thermochemical material collected in reference books and expressed in these units.
When one form of motion is transformed into another, the energies of the disappeared and appeared motion, expressed in different units, are equivalent to each other, i.e., the energy of the disappeared motion is in a constant quantitative relation to the energy of the motion that has arisen (the law of equivalent transformations of energy). This ratio does not depend on the energies of the two forms of motion and on the specific conditions under which the transition from one form of motion to another took place. So, when the energy of an electric current is converted into the energy of chaotic molecular motion, one joule of electrical energy always turns into 0.239 feces energy of molecular motion.
Thus, energy as a measure of the motion of matter always manifests itself in a qualitatively original form, corresponding to a given form of motion, and is expressed in the appropriate units of measurement. On the other hand, it quantitatively reflects the unity of all forms of movement, their mutual convertibility and the indestructibility of movement.
The above law of equivalent transformations of energy is a physical experimental law. The law of equivalent energy transformations can be expressed differently, namely in the form the law of conservation and transformation of energy: energy is neither created nor destroyed; in all processes and phenomena, the total energy of all parts of an isolated material system participating in this process does not increase or decrease, remaining constant.
The law of conservation and transformation of energy is universal in the sense that it is applicable to phenomena occurring in arbitrarily large bodies, representing an aggregate of a huge number of molecules, and to phenomena occurring with the participation of one or a few molecules.
For various forms of mechanical motion, the law of conservation of energy has long been expressed in a qualitative form (Descartes - 1640) and a quantitative form (Leibniz - 1697).
For the mutual transformations of heat and work (see below), the law of conservation of energy was proved as a natural science law by the studies of Yu. R. Mayer, G. Helmholtz and D.P. Joule, carried out in the forties of the XIX century.
Using the law of equivalent transformations, it is possible to express the energies of various forms of motion in units characteristic of one type of energy (one form of motion), and then perform operations of addition, subtraction, etc.
§ 2. Subject, method and limits of thermodynamics
Thermodynamics is one of the main branches of theoretical physics. Thermodynamics studies the laws of mutual transformations of various types of energy associated with the transfer of energy between bodies in the form of heat and work. Focusing its attention on heat and work as forms of energy transfer in a variety of processes, thermodynamics involves numerous energy connections and dependencies between various properties of a substance in its circle of consideration and gives very widely applicable generalizations called the laws of thermodynamics.
When establishing the basic thermodynamic laws, energy transformations (often very complex) occurring inside the body are usually not detailed. The types of energy inherent in the body in its given state are also not differentiated; the totality of all these types of energy is considered as a single internal energy of the system .
The subject matter of thermodynamics outlined above defines the method and boundaries of this science. The distinction between heat and work, taken as a starting point by thermodynamics, and the opposition of heat to work makes sense only for bodies consisting of many molecules, since for one molecule or for a set of a small number of molecules, the concepts of heat and work lose their meaning. Therefore, thermodynamics considers only bodies consisting of a large number of molecules, the so-called macroscopic systems moreover, thermodynamics in its classical form does not take into account the behavior and properties of individual molecules.
The thermodynamic method is also characterized by the fact that the object of study is a body or a group of bodies isolated from the material world into thermodynamic system (hereinafter referred to simply system).
The system has certain boundaries separating it from the outside world (environment).
The system is homogeneous , if each of its parameters has the same value in all parts of the system or continuously changes from point to point.
The system is heterogeneous , if it consists of several macroscopic (consisting in turn of many molecules) parts, separated from one another by visible interfaces. On these surfaces, some parameters change abruptly. Such, for example, is the system "solid salt - saturated aqueous salt solution - saturated water vapor." Here, at the boundaries of salt - solution and solution - vapor, the composition and density change abruptly.
Homogeneous parts of the system, separated from other parts by visible interfaces, are called phases . In this case, the set of individual homogeneous parts of the system that have the same physical and thermodynamic properties is considered to be one phase (for example, a set of crystals of one substance or a set of liquid droplets suspended in a gas and forming fog). Each phase of the system is characterized by its own equation of state.
A system that cannot exchange matter and energy with the environment (in the form of heat or work) is called isolated .
A system that can exchange matter and energy with the environment (in the form of heat or work) is called open.
A system that cannot exchange matter with the environment, but can exchange energy (in the form of heat or work) is called closed .
Thermodynamics studies the relationship between such measurable properties of a material system as a whole and its macroscopic parts (phases), such as temperature, pressure, mass, density and chemical composition of the phases included in the system, and some other properties, as well as the relationship between changes in these properties.
The set of properties studied by thermodynamics (the so-called thermodynamic parameters of the system) defines thermodynamic state of the system. A change in any thermodynamic properties (even if only one) leads to a change in the thermodynamic state of the system.
All processes occurring in nature can be divided into spontaneous (natural) and non-spontaneous.
Spontaneous processes These are processes that do not require external energy input. For example, the transfer of heat from a body with a higher temperature to a body with a lower temperature, the dissolution of salt in water, etc., proceed by themselves.
Non-spontaneous processes require energy from the outside for their flow, for example, the separation of air into nitrogen and oxygen.
Thermodynamics mainly considers such states of a system in which its parameters (temperature, pressure, electrostatic potential, etc.) do not change spontaneously with time and have the same value at all points in the volume of individual phases. Such states are called balanced.
One of the basic postulates of thermodynamics is the statement that the course of any spontaneous process ultimately brings the isolated system to an equilibrium state, when its properties will no longer change, i.e., equilibrium will be established in the system.
States characterized by uneven and time-varying distributions of temperature, pressure, and composition within phases are nonequilibrium. They are considered by the thermodynamics of non-equilibrium (irreversible) processes, in which, in addition to the basic thermodynamic laws, additional assumptions are used.
Thermodynamics, built on the basis of the basic laws of thermodynamics, which are considered as a generalization of experience, is often called classical or phenomenological thermodynamics. Thermodynamics provides the theoretical foundations for the theory of heat engines; this section is called technical thermodynamics. The study of chemical processes from a thermodynamic point of view is engaged in chemical thermodynamics, which is one of the main branches of physical chemistry.
§ 3. Heat and work
Changes in the forms of motion during its transition from one body to another and the corresponding transformations of energy are very diverse. The forms of the transition of motion itself and the transitions of energy connected with it can be divided into two groups.
The first group includes only one form of motion transition by chaotic collisions of molecules of two adjoining bodies, i.e. by conduction (and at the same time by radiation). The measure of the movement transmitted in this way is heat .
The second group includes various forms of movement transition, the common feature of which is the movement of macroscopic masses under the action of any external forces that have a directed character. Such are the rise of bodies in a gravitational field, the transition of a certain amount of electricity from a larger electrostatic potential to a smaller one, the expansion of a gas under pressure, etc. The general measure of the movement transmitted by such means is Job .
Heat and work characterize qualitatively and quantitatively two different forms of transmission of motion from one part of the material world to another.
The transmission of motion is a kind of complex motion of matter, the two main forms of which we distinguish. Heat and work are measures of these two complex forms of motion of matter, and they should be considered as types of energy.
The common property of heat and work is that they matter only during the time intervals in which these processes take place. In the course of such processes, in some bodies the movement in one form or another decreases and the corresponding energy decreases, while in other bodies the movement in the same or other forms increases and the corresponding types of energy increase.
We are not talking about the stock of heat or work in any body, but only about the heat and work of a known process. After its completion, there is no need to talk about the presence of heat or work in the bodies.
§ 4. Equivalence of heat and work
A constant equivalent ratio between heat and work during their mutual transitions was established in the classical experiments of D.P. Joule (1842-1867). A typical Joule experiment is as follows.
Joule device for determining the mechanical equivalent of heat.
Weights falling from a known height rotate a stirrer immersed in water in a calorimeter (a weight and a calorimeter with water constitute a thermodynamic system.) The rotation of the stirrer blades in water causes the water in the calorimeter to heat up; the corresponding rise in temperature is quantified.
After the specified process is completed, the system must be brought to its original state. This can be done through mental experience. The weights rise to their original height, while external work is expended, which increases the energy of the system. In addition, heat is removed from the calorimeter (transferred to the environment) by cooling it to the initial temperature. These operations return the system to its original state, i.e., all measurable properties of the system acquire the same values that they had in the initial state. The process during which the properties of the system changed, and at the end of which it returned to its original state, is called circular (cyclic) process or cycle .
The only result of the described cycle is the removal of work from the environment surrounding the system, and the transfer to this environment of the heat taken from the calorimeter.
Comparison of these two quantities, measured in the corresponding units, shows a constant relationship between them, independent of the size of the load, the size of the calorimeter, and the specific amounts of heat and work in different experiments.
It is advisable to write the heat and work in a cyclic process as the sum (integral) of infinitely small (elementary) heats Q and infinitesimal (elementary) jobs W, and the initial and final limits of integration coincide (cycle).
Then the equivalence of heat and work in a cyclic process can be written as follows:
 (I, 1)
(I, 1)
In equation (I, 1), the sign  denotes integration over a cycle. Coefficient constancy k
reflects the equivalence of heat and work ( k is the mechanical equivalent of heat). Equation (I, 1) expresses the law of conservation of energy for a particular, very important case of the transformation of work into heat.
denotes integration over a cycle. Coefficient constancy k
reflects the equivalence of heat and work ( k is the mechanical equivalent of heat). Equation (I, 1) expresses the law of conservation of energy for a particular, very important case of the transformation of work into heat.
In the studies of Joule, Rowland (1880), Miculescu (1892), and others, the methods of friction in metals, impact, direct conversion of the work of an electric current into heat, stretching of solids, etc. were used. k always constant within the experimental error.
In what follows, it is always assumed that work and heat, with the help of the coefficient k expressed in the same units (no matter what) and the coefficient k goes down.
§ 5. Internal energy
For a non-circular process, the equality (I, 1) is not observed, since the system does not return to its original state. Instead, the equalities for a non-circular process can be written (omitting the coefficient k):
 ≠
≠

Since the limits of integration are generally arbitrary, then for elementary quantities W And Q:
Q W,
Consequently:
Q – W 0
Denote the difference Q – W for any elementary thermodynamic process through dU:
dU Q – W (I, 2)
or for the final process:


 – (I, 2a)
– (I, 2a)
Returning to the circular process, we obtain (from Equation I, 1):
 =
=
 –
–
 = 0 (I, 3)
= 0 (I, 3)
Thus, the value dU is the total differential of some system state function. When the system returns to its original state (after a cyclic change), the value of this function acquires its original value.
System state functionU , defined by the equalities (I, 2) or (I, 2a) is calledinternal energy systems .
Obviously, expression (I, 2a) can be written as follows:
= U 2 – U 1 = ∆ U = – (I, 2b)
U 2 – U 1 = ∆U = Q – W
This reasoning substantiates empirically the presence of a certain function of the state of the system, which has the meaning of the total measure of all movements that the system has.
In other words, internal energy includes the translational and rotational energy of molecules, the vibrational energy of atoms and groups of atoms in a molecule, the energy of electron motion, intranuclear and other types of energy, i.e. the totality of all types of particle energy in the system, with the exception of the potential and kinetic energy of the system itself .
Let us assume that the cyclic process was carried out in such a way that after the system returned to its initial state, the internal energy of the system did not take the initial value, but increased. In this case, the repetition of circular processes would cause the accumulation of energy in the system. It would be possible to convert this energy into work and obtain work in this way not at the expense of heat, but “out of nothing”, since in a circular process work and heat are equivalent to each other, which is shown by direct experiments.
Inability to complete the specified build cycle perpetuum mobile (perpetuum mobile) of the first kind, that gives work without spending an equivalent amount of another type of energy, is proved by the negative result of thousands of years of human experience. This result leads to the same conclusion that we obtained in a particular but more rigorous form by analyzing Joule's experiments.
Let us formulate the result obtained once more. The total energy supply of the system (its internal energy) as a result of a cyclic process returns to its original value, i.e., the internal energy of a system in a given state has one definite value and does not depend on what changes the system underwent before coming to this state.
In other words, the internal energy of the system is a single-valued, continuous and finite function of the state of the system.
The change in the internal energy of the system is determined by expression (I, 2b); the expression (I, 3) is valid for a circular process. With an infinitesimal change in some properties (parameters) of the system, the internal energy of the system also changes infinitesimally. This is a property of a continuous function.
Within thermodynamics, there is no need to use a general definition of the concept of internal energy. A formal quantitative definition through expressions (I, 2) or (I, 2a) is sufficient for all further thermodynamic reasoning and conclusions.
Since the internal energy of the system is a function of its state, then, as already mentioned, the increase in internal energy with infinitesimal changes in the parameters of the system states is the total differential of the state function. Breaking the integral in equation (I, 3) into two integrals over the sections of the path from the state 1 up to the state 2 (path "a") (see Fig. I) and vice versa - from the state 2
advanced course physical chemistry 6th exam Before mastering the discipline "Advanced physical chemistry"should be... by physical chemistry. / Edited by V.V. Budanova, N.K. Vorobyov. – L.: Chemistry, 1986. - 352 p. Practical work on physical chemistry ...
Work program in the discipline: "Organic and physical chemistry" for the specialty 060601 Medical biochemistry, graduate qualification code (65 specialist) form of education (full-time)
Working programmIn the pulpit In the library 1 Organic and physical chemistry(organic chemistry, part I). V.A. Startseva, L.E. Nikitina, N.P. ... In the pulpit In the library 1 Organic and physical chemistry(organic chemistry, part I). V.A. Startseva, L.E. Nikitina, N.P. ...
Examination No. 2 in physical chemistry
DocumentExamination No. 2 on physical chemistry Option 2 What is the temperature .... Examination No. 2 on physical chemistry Option 3 List the physical and chemical quantities ... Examination No. 2 on physical chemistry Option 12 Determination electrodes. ...
Methodical manual for laboratory work No. 4 in the course of physical chemistry for full-time students of the Faculty of Chemical Technology and the Faculty of Building Materials Science
ToolkitVALUES OF THE EQUILIBRIUM CONSTANT physical chemistry often there is a laboratory work concerning ... p. 3. Petrov N.A., Cherepanov V.A. Yermishina Yu.A. Workshop on physical chemistry. Toolkit. Yekaterinburg: publishing house...
The program of the entrance exam in the specialty 02. 00. 04 "Physical Chemistry"
ProgramEquilibrium // M.: Metallurgy.-1988.-560s. Well physical chemistry/ ME AND. Gerasimov, V.P. Dreving, E.I. Ermin and others: under ... .- 1980.- 180s. Gorshkov B.I., Kuznetsov I.A. / Basics physical chemistry. 2nd ed. // M.: Publishing House of Moscow University...
1. Physical chemistry: purpose, tasks, research methods. Basic concepts of physical chemistry.
Phys. chemistry - the science of the laws of chemical processes and chemical. phenomena.
Subject of physical chemistry explanation of chem. phenomena based on more general laws of physics. Physical chemistry considers two main groups of issues:
1. Study of the structure and properties of a substance and its constituent particles;
2. The study of the processes of interaction of substances.
Physical chemistry aims to study the relationship between m / y chemical and physical phenomena. Knowledge of such relationships is necessary in order to study more deeply the chemical reactions that occur in nature and are used in technology. processes, control the depth and direction of the reaction. The main goal of the discipline Physical Chemistry is the study of general relationships and patterns of chemical. processes based on the fundamental principles of physics. Physical chemistry applies physical. theories and methods for chemical phenomena.
It explains WHY and HOW the transformations of substances occur: chem. reactions and phase transitions. WHY - chemical thermodynamics. AS - chemical kinetics.
Basic concepts of physical chemistry
The main object of chem. thermodynamics is a thermodynamic system. Thermodynamic system - any body or set of bodies capable of exchanging energy and matter with itself and with other bodies. Systems are divided into open, closed and isolated. open and I - the thermodynamic system exchanges with the external environment both in-tion and energy. Closed and I - a system in which there is no exchange of matter with the environment, but it can exchange energy with it. isolated and I -system volume remains constant and is deprived of the opportunity to exchange with the environment and energy and in-tion.
The system can be homogeneous (homogeneous) or heterogeneous (heterogeneous ). Phase - this is a part of the system, which in the absence of an external force field has the same composition at all its points and the same thermodynamic. St. you and separated from other parts of the system by the interface. The phase is always homogeneous, i.e. homogeneous, so a single-phase system is called homogeneous. A system consisting of several phases is called heterogeneous.
System properties are divided into two groups: extensive and intensive.
In thermodynamics, the concepts of equilibrium and reversible processes are used. equilibrium is a process that goes through a continuous series of equilibrium states. Reversible thermodynamic process is a process that can be carried out in reverse without leaving any changes in the system and environment.
2. I-th law of thermodynamics. Internal energy, heat, work.
First law of thermodynamics directly related to the law of conservation of energy. Based on this law, it follows that in any isolated system, the energy supply remains constant. Another formulation of the first law of thermodynamics follows from the law of conservation of energy - the impossibility of creating a perpetual motion machine (perpetuum mobile) of the first kind, which would produce work without spending energy on it. The formulation, especially important for chemical thermodynamics,
The first principle is its expression through the concept of internal energy: internal energy is a state function, i.e. its change does not depend on the path of the process, but depends only on the initial and final state of the system. Change in the internal energy of the system U can occur through heat exchange Q and work W with the environment. Then it follows from the law of conservation of energy that the heat Q received by the system from outside is spent on the increment of internal energy ΔU and the work W done by the system, i.e. Q=Δ U+W. Given at alignment is
mathematical expression of the first law of thermodynamics.
Ibeginning of thermodynamics its wording:
in any isolated system, the energy supply remains constant;
different forms of energy pass into each other in strictly equivalent quantities;
perpetual motion machine (perpetuum mobile) of the first kind is impossible;
internal energy is a state function, i.e. its change does not depend on the path of the process, but depends only on the initial and final state of the system.
analytic expression: Q = D U + W ; for an infinitesimal change in quantities d Q = dU + d W .
The 1st law of thermodynamics sets the ratio. m / y heat Q, work A and change int. system energy ΔU. Change int. The energy of the system is equal to the amount of heat communicated to the system minus the amount of work done by the system against external forces.
Equation (I.1) - mathematical notation of the 1st law of thermodynamics, equation (I.2) - for an infinitesimal change in comp. systems.
Int. energy-state function; this means that the change-e ext. energy ΔU does not depend on the transition path of the system from state 1 to state 2 and is equal to the difference between the values of ext. energies U2 and U1 in these states: (I.3)
Int. The energy of a system is the sum of the potential energy of the interaction. all particles of the body m / y and the kinetic energy of their movement (without taking into account the kinetic and potential energies of the system as a whole). Int. the energy of the system depends on the nature of the island, its mass and on the parameters of the state of the system. She's age. with an increase in the mass of the system, since it is an extensive property of the system. Int. energy is denoted by the letter U and is expressed in joules (J). In the general case, for a system with a quantity of 1 mol. Int. energy, like any thermodynamic. St. in the system, yavl-Xia function comp. Directly in the experiment, only changes in the internal energy. That is why in calculations they always operate with its change U2 –U1 = U.
All changes to the internal energies are divided into two groups. The 1st group includes only the 1st form of the transition of motion by chaotic collisions of the molecules of two adjoining bodies, i.e. by conduction (and at the same time by radiation). The measure of the movement transmitted in this way is heat. concept warmth associated with the behavior of a huge number of particles - atoms, molecules, ions. They are in constant chaotic (thermal) motion. Heat is a form of energy transfer. The second way to exchange energy is Job. This exchange of energy is due to the action performed by the system, or the action performed on it. Typically, work is denoted by the symbol W. Work, like heat, is not a function of the state of the system, so the value corresponding to infinitesimal work is denoted by the partial derivative symbol - W.
The science that explains chemical phenomena and establishes their patterns based on the general principles of physics. The name of the science Physical Chemistry was introduced by M.V. Lomonosov, who for the first time (1752 1753) formulated its subject and tasks and established one ... ... Big Encyclopedic Dictionary
PHYSICAL CHEMISTRY- PHYSICAL CHEMISTRY, “a science that explains, on the basis of provisions and experiments, the physical cause of what happens through chem. operations in complex bodies. This definition was given to her by the first physicochemist M.V. Lomonosov in a course read by ... Big Medical Encyclopedia
PHYSICAL CHEMISTRY, the science that studies the physical changes associated with CHEMICAL REACTIONS, as well as the relationship between physical properties and chemical composition. The main sections of physical chemistry THERMODYNAMICS, dealing with changes in energy in ... ... Scientific and technical encyclopedic dictionary
Physical chemistry- - a branch of chemistry in which the chemical properties of substances are studied on the basis of the physical properties of their constituent atoms and molecules. Modern physical chemistry is a broad interdisciplinary field bordering on various branches of physics… Encyclopedia of terms, definitions and explanations of building materials
PHYSICAL CHEMISTRY, explains chemical phenomena and establishes their laws on the basis of the general principles of physics. Includes chemical thermodynamics, chemical kinetics, the doctrine of catalysis, etc. The term physical chemistry was introduced by M.V. Lomonosov in 1753 ... Modern Encyclopedia
Physical chemistry- PHYSICAL CHEMISTRY, explains chemical phenomena and establishes their patterns based on the general principles of physics. It includes chemical thermodynamics, chemical kinetics, the doctrine of catalysis, etc. The term “physical chemistry” was introduced by M.V. Lomonosov in ... ... Illustrated Encyclopedic Dictionary
PHYSICAL CHEMISTRY- section of chem. science, studying chemistry. phenomena based on the principles of physics (see (1)) and physical. experimental methods. F. x. (like chemistry) includes the doctrine of the structure of matter, chem. thermodynamics and chemistry. kinetics, electrochemistry and colloidal chemistry, teaching ... ... Great Polytechnic Encyclopedia
Exist., number of synonyms: 1 physical (1) Dictionary of ASIS synonyms. V.N. Trishin. 2013 ... Synonym dictionary
physical chemistry- — EN physical chemistry A science dealing with the effects of physical phenomena on chemical properties. (Source: LEE) … … Technical Translator's Handbook
physical chemistry- - a science that explains chemical phenomena and establishes their patterns based on physical principles. Dictionary of Analytical Chemistry ... Chemical terms
Books
- Physical Chemistry, A. V. Artemov. The textbook was created in accordance with the Federal State Educational Standard in the areas of training of bachelors, providing for the study of the discipline `Physical Chemistry`.…
- Physical Chemistry, Yu. Ya. Kharitonov. The textbook outlines the basics of physical chemistry in accordance with the approximate program for the discipline "Physical and colloidal chemistry" for the specialty 060301 "Pharmacy". The publication is intended…
The beginning of physical chemistry was laid in the middle of the 18th century. The term "Physical chemistry", in the modern understanding of the methodology of science and questions of the theory of knowledge, belongs to M. V. Lomonosov, who for the first time read the "Course of True Physical Chemistry" to students of St. Petersburg University. In the preamble to these lectures, he gives the following definition: "Physical chemistry is a science that must, on the basis of the provisions and experiments of physical scientists, explain the reason for what happens through chemical operations in complex bodies." The scientist in the works of his corpuscular-kinetic theory of heat deals with issues that fully meet the above tasks and methods. This is precisely the nature of the experimental actions that serve to confirm individual hypotheses and provisions of this concept. M. V. Lomonosov followed these principles in many areas of his research: in the development and practical implementation of the “science of glass” founded by him, in various experiments devoted to confirming the law of conservation of matter and force (motion); - in works and experiments related to the doctrine of solutions - he developed an extensive program of research on this physical and chemical phenomenon, which is in the process of development to the present day.
This was followed by a break of more than a hundred years, and one of the first physicochemical studies in Russia in the late 1850s was started by D. I. Mendeleev.
The next course in physical chemistry was taught by N. N. Beketov at Kharkov University in 1865.
The first department of physical chemistry in Russia was opened in 1914 at the Faculty of Physics and Mathematics of St. Petersburg University, in the fall, a student of D.P. Konovalov, M.S. Vrevsky, began to read the compulsory course and practical classes in physical chemistry.
The first scientific journal intended to publish articles on physical chemistry was founded in 1887 by W. Ostwald and J. van't Hoff.
The subject of physical chemistry
Physical chemistry is the main theoretical foundation of modern chemistry, using the theoretical methods of such important sections of physics as quantum mechanics, statistical physics and thermodynamics, nonlinear dynamics, field theory, etc. It includes the doctrine of the structure of matter, including: the structure of molecules, chemical thermodynamics, chemical kinetics and catalysis. As separate sections in physical chemistry, electrochemistry, photochemistry, physical chemistry of surface phenomena (including adsorption), radiation chemistry, the theory of metal corrosion, physical chemistry of macromolecular compounds (see polymer physics), etc. are also distinguished. Very closely adjacent to physical chemistry and are sometimes considered as its independent sections of colloid chemistry, physico-chemical analysis and quantum chemistry. Most sections of physical chemistry have fairly clear boundaries in terms of objects and methods of research, in terms of methodological features and the apparatus used.
The difference between physical chemistry and chemical physics
The molecularity of an elementary reaction is the number of particles that, according to the experimentally established reaction mechanism, participate in an elementary act of chemical interaction. Monomolecular reactions- reactions in which a chemical transformation of one molecule occurs (isomerization, dissociation, etc.): H 2 S → H 2 + S Bimolecular reactions- reactions, the elementary act of which is carried out when two particles (identical or different) collide: CH 3 Br + KOH → CH 3 OH + KBr Trimolecular reactions- reactions, the elementary act of which is carried out when three particles collide: O 2 + NO + NO → 2NO 2 Reactions with a molecularity greater than three are unknown. For elementary reactions carried out at close concentrations of the starting substances, the values of molecularity and order of the reaction are the same. There is no clearly defined relationship between the concepts of molecularity and reaction order, since the reaction order characterizes the kinetic equation of the reaction, and molecularity characterizes the reaction mechanism. The main areas of chemical thermodynamics are:
Chemical thermodynamics is closely related to such branches of chemistry as
Physico-chemical analysisThe theory of physicochemical analysis is based on the principles of correspondence and continuity formulated by N. S. Kurnakov. The principle of continuity states that if new phases are not formed in the system or existing ones do not disappear, then with a continuous change in the parameters of the system, the properties of individual phases and the properties of the system as a whole change continuously. The correspondence principle states that each complex of phases corresponds to a certain geometric image on the composition-property diagram. Theory of reactivity of chemical compoundsHigh energy chemistry (HVE) studies the chemical reactions and transformations that occur in matter under the influence of nonthermal energy. The mechanisms and kinetics of such reactions and transformations are characterized by essentially nonequilibrium concentrations of fast, excited or ionized particles with an energy greater than the energy of their thermal motion and, in some cases, chemical bonding. Carriers of non-thermal energy acting on matter: accelerated electrons and ions, fast and slow neutrons, alpha and beta particles, positrons, muons, pions, atoms and molecules at supersonic speeds, electromagnetic radiation quanta, as well as pulsed electrical, magnetic and acoustic fields. The processes of high-energy chemistry are distinguished by time stages into physical, occurring in a time of femtoseconds or less, during which non-thermal energy is distributed unevenly in the medium and a “hot spot” is formed, physical-chemical, during which disequilibrium and inhomogeneity in the “hot spot” are manifested and, finally, chemical, in which the transformations of matter obey the laws of general chemistry. As a result, such ions and excited states of atoms and molecules are formed at room temperatures that cannot arise due to equilibrium processes. The external manifestation of CHE is the formation of ions and excited states of atoms and molecules at room temperatures, at which these particles cannot arise due to equilibrium processes. NE Ablesimov formulated a relaxation principle for controlling the properties of non-equilibrium physical and chemical systems. In the case when the relaxation times are much longer than the duration of the physical impact, it is possible to control the release of chemical forms, phases and, as a consequence, the properties of substances (materials), using information about the relaxation mechanisms in nonequilibrium condensed systems at the physicochemical stage of relaxation processes (including number and during operation). The main sections of the HVE
and others. laser chemistryThe ionizing ability is possessed by electromagnetic radiation (X-rays, γ-radiation, synchrotron radiation) and streams of accelerated particles (electrons, protons, neutrons, helions, heavy ions; fission fragments of heavy nuclei, etc.), the energy of which exceeds the ionization potential of atoms or molecules ( in most cases, lying in the range of 10-15 eV). Within the framework of radiation chemistry, some chemical processes are considered that are impossible using traditional chemical approaches. Ionizing radiation can greatly reduce the temperature of chemical reactions without the use of catalysts and initiators. History of radiation chemistry Radiation chemistry arose after the discovery of x-rays by W. Roentgen in 1895 and radioactivity by A. Becquerel in 1896, who were the first to observe radiation effects in photographic plates. The first works on radiation chemistry were carried out in 1899-1903 by the spouses M.Curie and P.Curie. In subsequent years, the greatest number of studies was devoted to the radiolysis of water and aqueous solutions. nuclear chemistryMain directions of nuclear chemistry:
ElectrochemistryElectrochemistry is a branch of chemical science that considers systems and interphase boundaries when an electric current flows through them, processes in conductors, on electrodes (from metals or semiconductors, including graphite) and in ionic conductors (electrolytes) are studied. Electrochemistry investigates processes |
The content of the article
CHEMISTRY PHYSICAL, a branch of chemistry that studies the chemical properties of substances based on the physical properties of their constituent atoms and molecules. Modern physical chemistry is a broad interdisciplinary field bordering on various branches of physics, biophysics, and molecular biology. It has many points of contact with such branches of chemical science as organic and inorganic chemistry.
A distinctive feature of the chemical approach (as opposed to the physical and biological) is that, along with the description of macroscopic phenomena, their nature is explained based on the properties of individual molecules and the interactions between them.
New instrumental and methodological developments in the field of physical chemistry are used in other branches of chemistry and related sciences, such as pharmacology and medicine. Examples include electrochemical methods, infrared (IR) and ultraviolet (UV) spectroscopy, laser and magnetic resonance techniques, which are widely used in therapy and for the diagnosis of various diseases.
The main sections of physical chemistry are traditionally considered: 1) chemical thermodynamics; 2) kinetic theory and statistical thermodynamics; 3) questions of the structure of molecules and spectroscopy; 4) chemical kinetics.
Chemical thermodynamics.
Chemical thermodynamics is directly related to the application of thermodynamics - the science of heat and its transformations - to the problem of chemical equilibrium. The essence of the problem is formulated as follows: if there is a mixture of reagents (system) and the physical conditions in which it is located (temperature, pressure, volume) are known, then what spontaneous chemical and physical processes can bring this system to equilibrium? The first law of thermodynamics states that heat is a form of energy and that the total energy of a system (together with its environment) remains unchanged. Thus, this law is one of the forms of the law of conservation of energy. According to the second law, a spontaneously occurring process leads to an increase in the total entropy of the system and its environment. Entropy is a measure of the amount of energy that a system cannot use to do useful work. The second law indicates the direction in which the reaction will go without any external influences. To change the nature of the reaction (for example, its direction), you need to expend energy in one form or another. Thus, it imposes strict limits on the amount of work that can be done as a result of the conversion of heat or chemical energy released in a reversible process.
We owe important achievements in chemical thermodynamics to J. Gibbs, who laid the theoretical foundation of this science, which made it possible to combine the results obtained by many researchers of the previous generation into a single whole. The approach developed by Gibbs does not make any assumptions about the microscopic structure of matter, but considers the equilibrium properties of systems at the macro level. This is why one can think that the first and second laws of thermodynamics are universal and will remain valid even when we learn much more about the properties of molecules and atoms.
Kinetic theory and statistical thermodynamics.
Statistical thermodynamics (as well as quantum mechanics) allows one to predict the equilibrium position for some reactions in the gas phase. With the help of the quantum mechanical approach, it is possible to describe the behavior of complex molecules of a number of substances that are in liquid and solid states. However, there are reactions whose rates cannot be calculated either within the framework of the kinetic theory or with the help of statistical thermodynamics.
A real revolution in classical statistical thermodynamics took place in the 1970s. New concepts such as universality (the notion that members of some broad classes of compounds have the same properties) and the principle of similarity (estimation of unknown quantities based on known criteria) have led to a better understanding of the behavior of liquids near the critical point, when the distinction between liquid and gas. Using a computer, the properties of simple (liquid argon) and complex (water and alcohol) liquids in a critical state were simulated. More recently, the properties of liquids such as liquid helium (whose behavior is perfectly described in the framework of quantum mechanics) and free electrons in molecular liquids have been comprehensively investigated using computer simulations (SUPERCONDUCTIVITY). This allowed a better understanding of the properties of ordinary liquids. Computer methods combined with the latest theoretical developments are intensively used to study the behavior of solutions, polymers, micelles (specific colloidal particles), proteins and ionic solutions. To solve problems of physical chemistry, in particular, to describe some properties of systems in a critical state and to study issues of high energy physics, the mathematical method of the renormalization group is increasingly being used.
The structure of molecules and spectroscopy.
Organic chemists of the 19th century. developed simple rules for determining the valency (ability to combine) of many chemical elements. For example, they found that the valence of carbon is 4 (one carbon atom can attach four hydrogen atoms to form a methane molecule CH 4), oxygen - 2, hydrogen - 1. Based on empirical ideas based on experimental data, assumptions were made about the spatial arrangement atoms in molecules (for example, the methane molecule has a tetrahedral structure, while the carbon atom is in the center of a triangular pyramid, and hydrogen is in its four vertices). However, this approach did not allow revealing the mechanism of formation of chemical bonds, and therefore, to estimate the size of molecules, to determine the exact distance between atoms.
Using spectroscopic methods developed in the 20th century, the structure of water molecules (H 2 O), ethane (C 2 H 6), and then much more complex molecules, such as proteins, was determined. The methods of microwave spectroscopy (EPR, NMR) and electron diffraction made it possible to establish the bond lengths, the angles between them (valence angles) and the mutual arrangement of atoms in simple molecules, and X-ray diffraction analysis - similar parameters for larger molecules that form molecular crystals. The compilation of catalogs of molecular structures and the use of simple concepts of valence laid the foundations of structural chemistry (L. Pauling was its pioneer) and made it possible to use molecular models to explain complex phenomena at the molecular level. If the molecules did not have a definite structure, or if the parameters of the C–C and C–H bonds in chromosomes were very different from those in the molecules of methane or ethane, then with the help of simple geometric models, J. Watson and F. Crick would not be able to build at the beginning 1950s for his famous double helix model of deoxyribonucleic acid (DNA). By studying the vibrations of atoms in molecules using IR and UV spectroscopy, it was possible to establish the nature of the forces that hold atoms in the composition of molecules, which, in turn, led to the idea of the presence of intramolecular motion and made it possible to study the thermodynamic properties of molecules ( see above). This was the first step towards determining the rates of chemical reactions. Further, spectroscopic studies in the UV region helped to establish the mechanism of chemical bond formation at the electronic level, which made it possible to describe chemical reactions based on the idea of the transition of reactants to an excited state (often under the action of visible or UV light). There was even a whole scientific field - photochemistry. Nuclear magnetic resonance (NMR) spectroscopy has made it possible for chemists to study individual stages of complex chemical processes and to identify active centers in enzyme molecules. This method also made it possible to obtain three-dimensional images of intact cells and individual organs. PHOTOCHEMISTRY.
Valency theory.
Using the empirical rules of valency developed by organic chemists, the periodic system of elements and Rutherford's planetary model of the atom, G. Lewis found that the key to understanding the chemical bond is the electronic structure of matter. Lewis came to the conclusion that a covalent bond is formed as a result of the socialization of electrons belonging to different atoms; in doing so, he proceeded from the idea that binding electrons are located on strictly defined electron shells. Quantum theory makes it possible to predict the structure of molecules and the nature of the covalent bonds formed in the most general case.
Our ideas about the structure of matter, which were formed due to the successes of quantum physics in the first quarter of the 20th century, can be summarized as follows. The structure of an atom is determined by the balance of electrical forces of repulsion (between electrons) and attraction (between electrons and a positively charged nucleus). Almost all the mass of an atom is concentrated in the nucleus, and its size is determined by the amount of space occupied by the electrons that revolve around the nuclei. Molecules consist of relatively stable nuclei held together by fast moving electrons, so that all chemical properties of substances can be explained in terms of the electrical interaction of elementary particles that make up atoms and molecules. Thus, the main provisions of quantum mechanics, concerning the structure of molecules and the formation of chemical bonds, create the basis for an empirical description of the electronic structure of matter, the nature of the chemical bond, and the reactivity of atoms and molecules.
With the advent of high-speed computers, it was possible to calculate (with a low but sufficient accuracy) the forces acting between atoms in small polyatomic molecules. The theory of valence, based on computer simulation, is currently a working tool for studying the structures, nature of chemical forces and reactions in cases where experiments are difficult or time consuming. This refers to the study of free radicals present in the atmosphere and flames or formed as reaction intermediates. There is hope that someday a theory based on computer calculations will be able to answer the question: how do chemical structures “calculate” their most stable state in a time of the order of picoseconds, while obtaining the corresponding estimates, at least in some approximation, requires a huge amount of machine time.
Chemical kinetics
deals with the study of the mechanism of chemical reactions and the determination of their rates. At the macroscopic level, the reaction can be represented as successive transformations, during which others are formed from one substance. For example, the seemingly simple transformation
H 2 + (1/2) O 2 → H 2 O
actually consists of several successive stages:
H + O 2 → OH + O
O + H 2 → HO + H
H + O 2 → HO 2
HO 2 + H 2 → H 2 O + OH
and each of them is characterized by its own rate constant k. S. Arrhenius suggested that the absolute temperature T and reaction rate constant k related by the ratio k = A exp(- E Act)/ RT, where BUT– pre-exponential factor (so-called frequency factor), E act - activation energy, R is the gas constant. For measuring k And T instruments are needed to track events that occur over a time of about 10–13 s, on the one hand, and over decades (and even millennia), on the other (geological processes); it is also necessary to be able to measure negligible concentrations of extremely unstable reagents. The task of chemical kinetics also includes the prediction of chemical processes occurring in complex systems (we are talking about biological, geological, atmospheric processes, combustion and chemical synthesis).
To study gas-phase reactions "in pure form" the method of molecular beams is used; in this case, molecules with strictly defined quantum states react with the formation of products that are also in certain quantum states. Such experiments provide information about the forces that cause certain reactions to occur. For example, in a molecular beam setup, even such small molecules as CH 3 I can be oriented in a given way and the collision rates in two “different” reactions can be measured:
K + ICH 3 → KI + CH 3
K + CH 3 I → KI + CH 3
where the CH 3 group is oriented differently with respect to the approaching potassium atom.
One of the issues that physical chemistry (as well as chemical physics) deals with is the calculation of reaction rate constants. Here, the transition state theory developed in the 1930s, which uses thermodynamic and structural parameters, is widely used. This theory, combined with the methods of classical physics and quantum mechanics, makes it possible to simulate the course of a reaction as if it were occurring under the conditions of an experiment with molecular beams. Experiments are being carried out on laser excitation of certain chemical bonds, which make it possible to test the correctness of the statistical theories of the destruction of molecules. Theories are being developed that generalize modern physical and mathematical concepts of chaotic processes (for example, turbulence). We are not so far from fully understanding the nature of both intra- and intermolecular interactions, revealing the mechanism of reactions occurring on surfaces with desired properties, and establishing the structure of the catalytic centers of enzymes and transition metal complexes. At the microscopic level, works on the formation kinetics of such complex structures as snowflakes or dendrites (crystals with a tree structure) can be noted, which stimulated the development of computer simulations based on simple models of the theory of nonlinear dynamics; this opens up prospects for creating new approaches to describing the structure and development of complex systems.