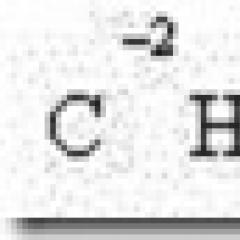
Iron strong or weak. Acids and bases
Before discussing the chemical properties of bases and amphoteric hydroxides, let's clearly define what it is?
1) Bases or basic hydroxides include metal hydroxides in the oxidation state +1 or +2, i.e. the formulas of which are written either as MeOH or as Me(OH) 2 . However, there are exceptions. So, the hydroxides Zn (OH) 2, Be (OH) 2, Pb (OH) 2, Sn (OH) 2 do not belong to the bases.
2) Amphoteric hydroxides include metal hydroxides in the oxidation state +3, +4, and, as exceptions, hydroxides Zn (OH) 2, Be (OH) 2, Pb (OH) 2, Sn (OH) 2. Metal hydroxides in the oxidation state +4 are not found in the USE assignments, therefore they will not be considered.
Chemical properties of bases
All bases are divided into:
Recall that beryllium and magnesium are not alkaline earth metals.
In addition to being soluble in water, alkalis also dissociate very well in aqueous solutions, while insoluble bases have a low degree of dissociation.
This difference in solubility and ability to dissociate between alkalis and insoluble hydroxides leads, in turn, to noticeable differences in their chemical properties. So, in particular, alkalis are more chemically active compounds and are often capable of entering into those reactions that insoluble bases do not enter into.
Reaction of bases with acids
Alkalis react with absolutely all acids, even very weak and insoluble ones. For example:
Insoluble bases react with almost all soluble acids, do not react with insoluble silicic acid:
It should be noted that both strong and weak bases with the general formula of the form Me (OH) 2 can form basic salts with a lack of acid, for example:
Interaction with acid oxides
Alkalis react with all acidic oxides to form salts and often water:
Insoluble bases are able to react with all higher acid oxides corresponding to stable acids, for example, P 2 O 5, SO 3, N 2 O 5, with the formation of medium salts:
Insoluble bases of the form Me (OH) 2 react in the presence of water with carbon dioxide exclusively with the formation of basic salts. For example:
Cu(OH) 2 + CO 2 = (CuOH) 2 CO 3 + H 2 O
With silicon dioxide, due to its exceptional inertness, only the strongest bases, alkalis, react. In this case, normal salts are formed. The reaction does not proceed with insoluble bases. For example:
Interaction of bases with amphoteric oxides and hydroxides
All alkalis react with amphoteric oxides and hydroxides. If the reaction is carried out by fusing an amphoteric oxide or hydroxide with a solid alkali, such a reaction leads to the formation of hydrogen-free salts:
If aqueous solutions of alkalis are used, then hydroxo complex salts are formed:
In the case of aluminum, under the action of an excess of concentrated alkali, instead of the Na salt, the Na 3 salt is formed:
The interaction of bases with salts
Any base reacts with any salt only if two conditions are met simultaneously:
1) solubility of starting compounds;
2) the presence of a precipitate or gas among the reaction products
For example:
Thermal stability of bases
All alkalis, except Ca(OH) 2 , are resistant to heat and melt without decomposition.
All insoluble bases, as well as slightly soluble Ca (OH) 2, decompose when heated. The highest decomposition temperature for calcium hydroxide is about 1000 o C:
Insoluble hydroxides have much lower decomposition temperatures. So, for example, copper (II) hydroxide decomposes already at temperatures above 70 o C:
Chemical properties of amphoteric hydroxides
Interaction of amphoteric hydroxides with acids
Amphoteric hydroxides react with strong acids:
Amphoteric metal hydroxides in the +3 oxidation state, i.e. type Me (OH) 3, do not react with acids such as H 2 S, H 2 SO 3 and H 2 CO 3 due to the fact that salts that could be formed as a result of such reactions are subject to irreversible hydrolysis to the original amphoteric hydroxide and corresponding acid:
Interaction of amphoteric hydroxides with acid oxides
Amphoteric hydroxides react with higher oxides, which correspond to stable acids (SO 3, P 2 O 5, N 2 O 5):
Amphoteric metal hydroxides in the +3 oxidation state, i.e. type Me (OH) 3, do not react with acid oxides SO 2 and CO 2.
Interaction of amphoteric hydroxides with bases
Of the bases, amphoteric hydroxides react only with alkalis. In this case, if an aqueous solution of alkali is used, then hydroxo complex salts are formed:
And when amphoteric hydroxides are fused with solid alkalis, their anhydrous analogues are obtained:
Interaction of amphoteric hydroxides with basic oxides
Amphoteric hydroxides react when fused with oxides of alkali and alkaline earth metals:
Thermal decomposition of amphoteric hydroxides
All amphoteric hydroxides are insoluble in water and, like any insoluble hydroxides, decompose when heated to the corresponding oxide and water.
We have defined hydrolysis remembered some facts about salts. Now we will discuss strong and weak acids and find out that the "scenario" of hydrolysis depends precisely on which acid and which base formed this salt.
← Hydrolysis of salts. Part I
Strong and weak electrolytes
Let me remind you that all acids and bases can be conditionally divided into strong And weak. Strong acids (and, in general, strong electrolytes) dissociate almost completely in aqueous solution. Weak electrolytes decompose into ions to a small extent.
Strong acids include:
- H 2 SO 4 (sulfuric acid),
- HClO 4 (perchloric acid),
- HClO 3 (chloric acid),
- HNO 3 (nitric acid),
- HCl (hydrochloric acid),
- HBr (hydrobromic acid),
- HI (hydroiodic acid).
The following is a list of weak acids:
- H 2 SO 3 (sulphurous acid),
- H 2 CO 3 (carbonic acid),
- H 2 SiO 3 (silicic acid),
- H 3 PO 3 (phosphorous acid),
- H 3 PO 4 (orthophosphoric acid),
- HClO 2 (chlorous acid),
- HClO (hypochlorous acid),
- HNO 2 (nitrous acid),
- HF (hydrofluoric acid),
- H 2 S (hydrosulfuric acid),
- most organic acids, e.g. acetic (CH 3 COOH).
Naturally, it is impossible to list all the acids that exist in nature. Only the most "popular" ones are listed. It should also be understood that the division of acids into strong and weak is rather arbitrary.
Things are much simpler with strong and weak bases. You can use the solubility table. All strong bases are soluble in base water, except for NH 4 OH. These substances are called alkalis (NaOH, KOH, Ca (OH) 2, etc.)
Weak bases are:
- all water-insoluble hydroxides (eg Fe(OH) 3 , Cu(OH) 2 etc.),
- NH 4 OH (ammonium hydroxide).
Salt hydrolysis. general facts
It may seem to those reading this article that we have already forgotten about the main topic of the conversation, and have gone somewhere to the side. This is not true! Our conversation about acids and bases, about strong and weak electrolytes is directly related to the hydrolysis of salts. Now you will be convinced of it.
So let me give you the basic facts:
- Not all salts undergo hydrolysis. Exist hydrolytically stable compounds such as sodium chloride.
- Hydrolysis of salts can be complete (irreversible) and partial (reversible).
- During the hydrolysis reaction, an acid or base is formed, the acidity of the medium changes.
- The fundamental possibility of hydrolysis, the direction of the corresponding reaction, its reversibility or irreversibility are determined acid power And by force of foundation that form this salt.
- Depending on the strength of the corresponding acid and resp. bases, all salts can be divided into 4 groups. Each of these groups has its own "scenario" of hydrolysis.
Example 4. Salt NaNO 3 is formed by a strong acid (HNO 3) and a strong base (NaOH). Hydrolysis does not occur, no new compounds are formed, the acidity of the medium does not change.
Example 5. Salt NiSO 4 is formed by a strong acid (H 2 SO 4) and a weak base (Ni (OH) 2). Hydrolysis occurs at the cation, during the reaction an acid and a basic salt are formed.
Example 6. Potassium carbonate is formed from a weak acid (H 2 CO 3) and a strong base (KOH). Anion hydrolysis, formation of alkali and acid salt. Alkaline solution.
Example 7. Aluminum sulfide is formed by a weak acid (H 2 S) and a weak base (Al (OH) 3). Hydrolysis occurs both at the cation and at the anion. irreversible reaction. During the process, H 2 S and aluminum hydroxide are formed. The acidity of the environment changes slightly.
Try it yourself:
Exercise 2. What type are the following salts: FeCl 3 , Na 3 PO 3 , KBr, NH 4 NO 2 ? Do these salts undergo hydrolysis? Cation or anion? What is formed during the reaction? How does the acidity of the environment change? The reaction equations can not yet be written down.
It remains for us to sequentially discuss 4 groups of salts and give a specific "scenario" of hydrolysis for each of them. In the next part, we will start with salts formed from a weak base and a strong acid.
To understand how the hydrolysis of salts proceeds in their aqueous solutions, we first give a definition of this process.
Definition and features of hydrolysis
This process involves the chemical action of water ions with salt ions, as a result a weak base (or acid) is formed, and the reaction of the medium also changes. Any salt can be represented as a chemical reaction product of a base and an acid. Depending on what their strength is, there are several options for the course of the process.
Types of hydrolysis
In chemistry, three types of reactions between salt and water cations are considered. Each process is carried out with a change in the pH of the medium, so it is expected to use different types of indicators to determine the pH value. For example, purple litmus is used for an acidic reaction, phenolphthalein is suitable for an alkaline reaction. Let us analyze in more detail the features of each hydrolysis variant. Strong and weak bases can be determined from the solubility table, and the strength of acids can be determined from the table.

Hydrolysis by cation
As an example of such a salt, consider ferric chloride (2). Iron(2) hydroxide is a weak base, while hydrochloric acid is a strong base. In the process of interaction with water (hydrolysis), the formation of a basic salt (iron hydroxochloride 2) occurs, and hydrochloric acid is also formed. An acidic environment appears in the solution, it can be determined using blue litmus (pH less than 7). In this case, the hydrolysis itself proceeds through the cation, since a weak base is used.
Let us give one more example of hydrolysis proceeding for the described case. Consider the magnesium chloride salt. Magnesium hydroxide is a weak base, while hydrochloric acid is a strong base. In the process of interaction with water molecules, magnesium chloride turns into a basic salt (hydroxochloride). Magnesium hydroxide, whose general formula is M(OH) 2 , is slightly soluble in water, but strong hydrochloric acid makes the solution acidic.

Anion hydrolysis
The next variant of hydrolysis is typical for a salt, which is formed by a strong base (alkali) and a weak acid. As an example for this case, consider sodium carbonate.
This salt contains a strong sodium base and a weak carbonic acid. Interaction with water molecules proceeds with the formation of an acid salt - sodium bicarbonate, that is, hydrolysis occurs along the anion. In addition, the solution is formed which gives the solution an alkaline environment.
Let's give another example for this case. Potassium sulfite is a salt that is formed by a strong base - caustic potassium, as well as a weak one. In the process of interaction with water (during hydrolysis), potassium hydrosulfite (acid salt) and potassium hydroxide (alkali) are formed. The environment in the solution will be alkaline, it can be confirmed using phenolphthalein.

Complete hydrolysis
The salt of a weak acid and a weak base undergoes complete hydrolysis. Let's try to find out what is its peculiarity, and what products will be formed as a result of this chemical reaction.
Let us analyze the hydrolysis of a weak base and a weak acid using aluminum sulfide as an example. This salt is formed by aluminum hydroxide, which is a weak base, as well as a weak hydrosulphuric acid. When interacting with water, complete hydrolysis is observed, as a result of which gaseous hydrogen sulfide is formed, as well as aluminum hydroxide in the form of a precipitate. Such an interaction occurs both in the cation and in the anion; therefore, this hydrolysis option is considered complete.
Magnesium sulfide can also be cited as an example of the interaction of this type of salt with water. This salt contains magnesium hydroxide, its formula is Mg (OH) 2. It is a weak base, insoluble in water. In addition, there is hydrosulfide acid inside magnesium sulfide, which is weak. When interacting with water, complete hydrolysis occurs (according to the cation and anion), as a result of which magnesium hydroxide is formed in the form of a precipitate, and hydrogen sulfide is also released in the form of a gas.
If we consider the hydrolysis of a salt, which is formed by a strong acid and a strong base, then it should be noted that it does not proceed. The medium in solutions of salts such as potassium chloride remains neutral.

Conclusion
Strong and weak bases, acids that form salts, affect the result of hydrolysis, the reaction of the medium in the resulting solution. Similar processes are widespread in nature.
Hydrolysis is of particular importance in the chemical transformation of the earth's crust. It contains metal sulfides, which are sparingly soluble in water. As their hydrolysis occurs, the formation of hydrogen sulfide, its release in the process of volcanic activity to the surface of the earth.
Silicate rocks, when converted to hydroxides, cause gradual destruction of rocks. For example, a mineral such as malachite is a product of the hydrolysis of copper carbonates.
An intensive process of hydrolysis also occurs in the oceans. and calcium, which are carried out by water, have a slightly alkaline environment. Under such conditions, the process of photosynthesis in marine plants proceeds well, and marine organisms develop more intensively.
Oil contains impurities of water and salts of calcium and magnesium. In the process of heating oil, they interact with water vapor. During hydrolysis, hydrogen chloride is formed, the interaction of which with the metal causes the destruction of equipment.
Salt hydrolysis" - To form an idea of chemistry as a productive force of society. Acetic acid CH3COOH is the oldest of the organic acids. In acids - carboxyl groups, But all the acids here are weak.
All acids, their properties and bases are divided into strong and weak. For example, you cannot make a concentrated solution of a weak acid or a dilute solution of a strong base. Our water in this case plays the role of a base, as it receives a proton from hydrochloric acid. Acids that dissociate completely in aqueous solutions are called strong acids.
For oxides hydrated with an indefinite number of water molecules, for example, Tl2O3 n H2O, it is unacceptable to write formulas like Tl(OH)3. Calling such compounds hydroxides is also not recommended.
For bases, one can quantify their strength, that is, the ability to split off a proton from an acid. All bases are solids with different colors. Attention! Alkalis are very caustic substances. If it comes into contact with the skin, alkali solutions cause severe long-healing burns, if they get into the eyes, they can cause blindness. When roasting cobalt minerals containing arsenic, volatile toxic arsenic oxide is released.
These properties of the water molecule are already known to you. II) and a solution of acetic acid. HNO2) - only one proton.
All bases are solids that have different colors. 1. They act on indicators. Indicators change their color depending on the interaction with different chemicals. When interacting with bases, they change their color: the methyl orange indicator turns yellow, the litmus indicator turns blue, and phenolphthalein becomes fuchsia.
Cool the containers, for example by placing them in a vessel filled with ice. Three solutions will remain clear, and the fourth will quickly become cloudy, a white precipitate will begin to fall out. This is where the barium salt is located. Set this container aside. You can quickly determine barium carbonate in another way. This is fairly easy to make, all you need are porcelain evaporating cups and a spirit lamp. If it is a lithium salt, the color will be bright red. By the way, if barium salt were tested in the same way, the color of the flame should have been green.
An electrolyte is a substance that in the solid state is a dielectric, that is, does not conduct electric current, however, in a dissolved or molten form it becomes a conductor. Remember that the degree of dissociation and, accordingly, the strength of the electrolyte depend on many factors: the nature of the electrolyte itself, the solvent, and the temperature. Therefore, this division itself is to a certain extent conditional. After all, the same substance can, under different conditions, be both a strong electrolyte and a weak one.
Hydrolysis does not occur, no new compounds are formed, the acidity of the medium does not change. How does the acidity of the environment change? The reaction equations can not yet be written down. It remains for us to sequentially discuss 4 groups of salts and for each of them give a specific "scenario" of hydrolysis. In the next part, we will start with salts formed from a weak base and a strong acid.
After reading the article, you will be able to separate substances into salts, acids and bases. H solution, what are the general properties of acids and bases. If they mean the definition of a Lewis acid, then in the text such an acid is called a Lewis acid.
The lower this value, the stronger the acid. Strong or weak - this is needed in the reference book of Ph.D. watch, but you need to know the classics. Strong acids are acids that can displace the anion of another acid from the salt.
ELECTROLYTES Substances whose solutions or melts conduct electricity.
NON-ELECTROLYTES Substances whose solutions or melts do not conduct electricity.
Dissociation- decomposition of compounds into ions.
Degree of dissociation is the ratio of the number of molecules dissociated into ions to the total number of molecules in the solution.
STRONG ELECTROLYTES when dissolved in water, they almost completely dissociate into ions.
When writing the equations of dissociation of strong electrolytes put an equal sign.
Strong electrolytes include:
Soluble salts ( see solubility table);
Many inorganic acids: HNO 3, H 2 SO 4, HClO 3, HClO 4, HMnO 4, HCl, HBr, HI ( Look acids-strong electrolytes in the solubility table);
Bases of alkali (LiOH, NaOH, KOH) and alkaline earth (Ca (OH) 2, Sr (OH) 2, Ba (OH) 2) metals ( see strong electrolyte bases in the solubility table).
WEAK ELECTROLYTES in aqueous solutions only partially (reversibly) dissociate into ions.
When writing the dissociation equations for weak electrolytes, the sign of reversibility is put.
Weak electrolytes include:
Almost all organic acids and water (H 2 O);
Some inorganic acids: H 2 S, H 3 PO 4, HClO 4, H 2 CO 3, HNO 2, H 2 SiO 3 ( Look acids-weak electrolytes in the solubility table);
Insoluble metal hydroxides (Mg (OH) 2, Fe (OH) 2, Zn (OH) 2) ( see basescweak electrolytes in the solubility table).
The degree of electrolytic dissociation is influenced by a number of factors:
the nature of the solvent and electrolyte: strong electrolytes are substances with ionic and covalent strongly polar bonds; good ionizing ability, i.e. the ability to cause dissociation of substances, have solvents with a high dielectric constant, the molecules of which are polar (for example, water);
temperature: since dissociation is an endothermic process, an increase in temperature increases the value of α;
concentration: when the solution is diluted, the degree of dissociation increases, and with increasing concentration, it decreases;
stage of the dissociation process: each subsequent stage is less effective than the previous one, approximately 1000–10,000 times; for example, for phosphoric acid α 1 > α 2 > α 3:
H3PO4⇄Н++H2PO−4 (first stage, α 1),
H2PO−4⇄H++HPO2−4 (second stage, α 2),
НPO2−4⇄Н++PO3−4 (third stage, α 3).
For this reason, in a solution of this acid, the concentration of hydrogen ions is the highest, and the concentration of PO3−4 phosphate ions is the lowest.
1. Solubility and the degree of dissociation of a substance are not related to each other. For example, a weak electrolyte is acetic acid, which is highly (unrestrictedly) soluble in water.
2. A solution of a weak electrolyte contains less than others those ions that are formed at the last stage of electrolytic dissociation
The degree of electrolytic dissociation is also affected by addition of other electrolytes: e.g. degree of dissociation of formic acid
HCOOH ⇄ HCOO − + H+
decreases if a little sodium formate is added to the solution. This salt dissociates to form formate ions HCOO − :
HCOONa → HCOO − + Na +
As a result, the concentration of HCOO– ions in the solution increases, and according to the Le Chatelier principle, an increase in the concentration of formate ions shifts the equilibrium of the formic acid dissociation process to the left, i.e. the degree of dissociation decreases.
Ostwald dilution law- ratio expressing the dependence of the equivalent electrical conductivity of a dilute solution of a binary weak electrolyte on the concentration of the solution:
Here, is the dissociation constant of the electrolyte, is the concentration, and are the values of the equivalent electrical conductivity at concentration and at infinite dilution, respectively. The ratio is a consequence of the law of mass action and equality
where is the degree of dissociation.
The Ostwald dilution law was developed by W. Ostwald in 1888 and confirmed by him experimentally. The experimental establishment of the correctness of the Ostwald dilution law was of great importance for substantiating the theory of electrolytic dissociation.
Electrolytic dissociation of water. Hydrogen indicator pH Water is a weak amphoteric electrolyte: H2O H+ + OH- or, more precisely: 2H2O \u003d H3O + + OH- The dissociation constant of water at 25 ° C is: can be considered constant and equal to 55.55 mol / l (water density 1000 g / l, mass 1 l 1000 g, amount of water substance 1000g: 18g / mol \u003d 55.55 mol, C \u003d 55.55 mol: 1 l \u003d 55 .55 mol/l). Then This value is constant at a given temperature (25 ° C), it is called the ion product of water KW: The dissociation of water is an endothermic process, therefore, with an increase in temperature, in accordance with the Le Chatelier principle, dissociation increases, the ion product increases and reaches a value of 10-13 at 100 ° C. In pure water at 25°C, the concentrations of hydrogen and hydroxyl ions are equal to each other: = = 10-7 mol/l Solutions in which the concentrations of hydrogen and hydroxyl ions are equal to each other are called neutral. If acid is added to pure water, the concentration of hydrogen ions will increase and become more than 10-7 mol / l, the medium will become acidic, while the concentration of hydroxyl ions will instantly change so that the ion product of water retains its value of 10-14. The same thing will happen when alkali is added to pure water. The concentrations of hydrogen and hydroxyl ions are related to each other through the ion product, therefore, knowing the concentration of one of the ions, it is easy to calculate the concentration of the other. For example, if = 10-3 mol/l, then = KW/ = 10-14/10-3 = 10-11 mol/l, or if = 10-2 mol/l, then = KW/ = 10-14 /10-2 = 10-12 mol/l. Thus, the concentration of hydrogen or hydroxyl ions can serve as a quantitative characteristic of the acidity or alkalinity of the medium. In practice, it is not the concentrations of hydrogen or hydroxyl ions that are used, but the hydrogen pH or hydroxyl pOH indicators. The hydrogen index pH is equal to the negative decimal logarithm of the concentration of hydrogen ions: pH = - lg The hydroxyl index pOH is equal to the negative decimal logarithm of the concentration of hydroxyl ions: pOH = - lg It is easy to show by pronouncing the ionic product of water that pH + pOH = 14 the medium is neutral, if less than 7 - acidic, and the lower the pH, the higher the concentration of hydrogen ions. pH greater than 7 - alkaline environment, the higher the pH, the higher the concentration of hydroxyl ions.