capillary phenomena. Surface tension
The surface layer of the liquid has special properties. Liquid molecules in this layer are in close proximity to another phase - gas. A molecule located near the liquid-gas interface has nearest neighbors on one side only, so the sum of all the forces acting on this molecule gives the resultant directed inside the liquid. Therefore, any liquid molecule located near the free surface has an excess of potential energy compared to the molecules inside.
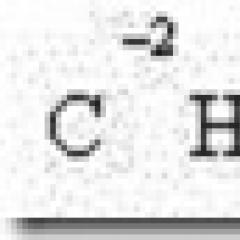
In order to transfer a molecule from the bulk of the liquid to the surface, work must be done. When the surface of a certain volume of liquid increases, the internal energy of the liquid increases. This component of the internal energy is proportional to the surface area of the liquid and is called the surface energy. The value of the surface energy depends on the forces of molecular interaction and the number of nearest neighboring molecules. For different substances, the surface energy takes on different values. The energy of the surface layer of a liquid is proportional to its area: E= σ S
The magnitude of the force F acting per unit length of the surface boundary determines the surface tension of the liquid: σ = F/ L; σ- liquid surface tension coefficient, N/m.
The easiest way to capture the nature of surface tension forces is to observe the formation of a drop at a loosely closed tap. Look carefully at how the drop gradually grows, a narrowing is formed - the neck and the drop comes off. The surface layer of water behaves like a stretched elastic film.
You can carefully place the sewing needle on the surface of the water. The surface film will bend and prevent the needle from sinking.

For the same reason, light insects - water striders can quickly glide over the surface of the water. The deflection of the film does not allow water to pour out, carefully poured into a fairly frequent sieve. A fabric is the same sieve formed by interlacing threads. Surface tension makes it very difficult for water to seep through, and therefore the fabric does not get wet instantly. Due to the forces of surface tension, foam is formed.
Change in surface tension
When a liquid comes into contact with a solid, the phenomenonwetting or non-wetting. If the forces of interaction between the molecules of the liquid and the solid are greater than between the molecules of the liquid, then the liquid spreads over the surface of the solid, i.e. wets and vice versa, if the interaction forces between the molecules of the liquid are greater than between the molecules of the liquid and the solid, then the liquid collects in a drop and does not wet the surface of the liquid.
capillary phenomena.
In nature, there are often bodies that have a porous structure (permeated with many small channels). Paper, leather, wood, soil, and many building materials have this structure. Water or other liquid, falling on such a solid body, can be absorbed into it, rising up to a great height. This is how moisture rises in the stems of plants, kerosene rises through the wick, and the fabric absorbs moisture. Such phenomena are called capillaries.

In a narrow cylindrical tube, the wetting liquid rises up due to the forces of molecular interaction, taking on a concave shape. An additional upward pressure appears under the concave surface, and therefore the liquid level in the capillary is higher than the level of the free surface. A non-wetting liquid takes on a convex surface. Under the convex surface of the liquid, a reverse additional downward pressure arises, so that the level of the liquid with a convex meniscus is lower than the level of the free surface.
The value of additional pressure is equal to p= 2 σ / R
The liquid in the capillary rises to such a height that the pressure of the liquid column balances the excess pressure. The liquid rise height in the capillary is: h = 2 σ / ρgr

Adsorption - a phenomenon similar to wetting, is observed when the solid and gaseous phases come into contact. If the forces of interaction between the molecules of a solid and gas are large, then the body is covered with a layer of gas molecules. Porous substances have a large adsorption capacity. The property of activated carbon to adsorb a large amount of gas is used in gas masks, in the chemical industry, and in medicine.
The value of surface tension
The concept of surface tension was first introduced by J. Segner (1752). In the 1st half of the 19th century. on the basis of the concept of surface tension, a mathematical theory of capillary phenomena was developed (P. Laplace, S. Poisson, K. Gauss, A.Yu. Davidov). In the 2nd half of the 19th century. J. Gibbs developed the thermodynamic theory of surface phenomena, in which surface tension plays a decisive role. Among the current topical problems is the development of the molecular theory of surface tension of various liquids, including molten metals. Surface tension forces play a significant role in natural phenomena, biology, medicine, various modern technologies, printing, engineering, and in the physiology of our body. Without these powers, we would not be able to write with ink. An ordinary pen would not scoop up ink from an inkwell, but an automatic one would immediately make a big blot, emptying its entire reservoir. It would be impossible to soap your hands: the foam would not form. The water regime of the soil would be disturbed, which would be disastrous for plants. Important functions of our body would suffer. The manifestations of surface tension forces are so diverse that it is not even possible to list them all.
In medicine, the dynamic and equilibrium surface tension of venous blood serum is measured, which can be used to diagnose a disease and control the treatment being carried out. It has been found that water with low surface tension is biologically more accessible. It enters into molecular interactions more easily, then the cells will not have to spend energy to overcome surface tension.
The volume of printing on polymer films is constantly growing due to the rapid development of the packaging industry, the high demand for consumer goods in colorful polymer packaging. An important condition for the competent implementation of such technologies is the precise definition of the conditions for their use in printing processes. In printing, processing plastic before printing is necessary so that the paint falls on the material. The reason is the surface tension of the material. The result is determined by how the liquid wets the surface of the product. Wetting is considered optimal when a drop of liquid remains where it was applied. In other cases, the liquid may roll into a drop, or, conversely, spread. Both cases equally lead to negative results during ink transfer.
Some conclusions:
1. A liquid may or may not wet a solid.2. The coefficient of surface tension depends on the type of liquid.
3. Surface tension coefficient depends on temperature.T σ ↓
4. The height of liquid rise in a capillary depends on its diameter. d h ↓
5. The force of surface tension depends on the length of the free surface of the liquid. lF

CAPILLARY PHENOMENA
CAPILLARY PHENOMENA
Phys. phenomena caused by surface tension at the interface of immiscible media. To K. I. usually include phenomena in liquid media caused by the curvature of their surface, which borders on another liquid, gas, or its own vapor.
The curvature of the surface leads to the appearance of additives in the liquid. capillary pressure Ar, the value of which is associated with cf. curvature r of the surface by the Laplace equation:
The movement of fluid in the capillaries can be caused by the difference in capillary pressure resulting from the expansion. curvature of the liquid surface. The liquid flow is directed towards lower pressure: for wetting liquids, towards the meniscus with a smaller radius of curvature (Fig. 2a).
Reduced, in accordance with the Kelvin equation, vapor pressure over the wetting meniscus yavl. cause capillary condensation of liquids in thin pores.
Negative capillary pressure exerts a constricting effect on the liquid-restricting walls (Fig. 2b).

Rice. 2. a - liquids in a capillary under the action of a difference in capillary pressures (r1>r2); b - the contracting effect of capillary pressure (eg, in a capillary with elastic walls).
This can lead to meaning. volumetric deformation of highly dispersed systems and porous bodies - capillary contraction. So, for example, the ongoing growth of capillary pressure during drying leads to a signifi- cant shrinkage of materials.
Many properties of dispersed systems (permeability, strength, liquid absorption) mean. To a certain extent are caused by K. Ya., since in the thin pores of these bodies high capillary pressures are realized.
K. i. were first discovered and studied by Leonardo da Vinci (1561), B. Pascal (17th century), and J. Zhuren (Dzhurin, 18th century) in experiments with capillary tubes. Theory K. I. developed in the works of P. Laplace (1806), T. Young (Young, 1805), J. W. Gibbs (1875) and I. S. Gromeka (1879, 1886).
Physical Encyclopedic Dictionary. - M.: Soviet Encyclopedia. . 1983 .
CAPILLARY PHENOMENA
-
a set of phenomena caused by the action of interfacial surface tension at the interface of immiscible media; to K. i. usually include phenomena in liquids caused by the curvature of their surface, bordering on another liquid, gas, or proper. ferry. K. Ya. is a special case of surface phenomena. the force of gravity. So, for example, when crushing a liquid in a gas (or a gas in a liquid), droplets (bubbles) spherical are formed. forms. The properties of systems containing a large number of drops or bubbles (emulsions, liquid aerosols, foams) and the conditions for their formation are largely determined by the curvature of the surface of these formations, that is, K. I. The big role of K. I. They also play in nucleation during vapor condensation, liquid boiling, and crystallization. liquid wetting of this surface. If it takes place, i.e., liquids 1 (Fig. 1) interact more strongly with the surface of a solid body 3 ,
than with molecules of another liquid (or gas) 2 ,
then, under the influence of the difference in the forces of intermolecular interaction, the liquid rises along the wall of the vessel and the section of the surface of the liquid adjacent to the solid body will be curved. Hydrostatic the pressure caused by the rise in the liquid level is balanced capillary pressure - pressure difference above and below a curved surface, the value of which is related to the local curvature of the liquid surface.
where r 1 and r 2 are the densities of liquid 1 and gas 2, s 12 is the interfacial tension, g- gravitational acceleration, r is the radius of the average curvature of the meniscus surface (1 / r \u003d 1 / R 1 +1 / R 2, where R 1 and R 2 are the radii of curvature of the meniscus in two mutually perpendicular sectional planes). For wetting liquid r<0 и h 0 >0. A non-wetting liquid forms a convex meniscus, the capillary pressure under the Crimea is positive, which leads to the lowering of the liquid in the capillary below the level of the free surface of the liquid (h 0<0). Радиус кривизны rсвязан с радиусом капилляра r к соотношением r=-r к /cosq, где q - краевой угол, образуемый поверхностью жидкости со стенками капилляра. а - величину, характеризующую размеры системы L<а, при к-рых становятся существенными К. я.: ![]() For water at a temperature of 20 ° C, a \u003d 0.38, see capillary condensation, evaporation and dissolution processes in the presence of a curved surface. For capillary absorption, an important characteristic is its v, determined by the value of capillary pressure and viscous resistance to fluid flow in the capillary. Speed v changes with absorption time t, and for a vertical capillary
For water at a temperature of 20 ° C, a \u003d 0.38, see capillary condensation, evaporation and dissolution processes in the presence of a curved surface. For capillary absorption, an important characteristic is its v, determined by the value of capillary pressure and viscous resistance to fluid flow in the capillary. Speed v changes with absorption time t, and for a vertical capillary
where h(t) - position of the meniscus at time t(Fig. 1), h - coefficient. fluid viscosity. When absorbed into a horizontal capillary
At v>10 -3 cm/s, one should take into account the possible dependence of the contact angle q on v, and in some cases - the viscous resistance of the gas (or other liquid) displaced from the capillary. The rate of capillary absorption plays a role in the water supply of plants, the movement of fluid in soils, and other porous bodies. Capillary impregnation is one of the most common chemical processes. technology. fluctuations in the thickness of thin layers of liquid (jet, film) - is the cause of their instability in relation to the state of drops or capillary condensate. 

For wetting liquids, the fluid flow is directed towards the meniscus with a smaller radius of curvature (i.e., towards lower pressure). The reason for capillary movement can be not only the gradient of curvature, but also the gradient of the surface tension of the liquid. Thus, the temperature gradient leads to a difference in surface tension and, consequently, to a difference in capillary pressure in the liquid (thermocapillary flow). This also explains the liquid droplets and gas bubbles in an unevenly heated medium: under the influence of the surface tension gradient, the surface of the bubbles or drops begins to move. A similar effect is also observed when s 12 changes during adsorption surfactants(SAW): SAW reduce s 12 and the liquid moves in the direction where the surfactant on the surface of the liquid is less (Marangoni-Gibbs effect). The curvature of the phase interface leads to a change in the value of the equilibrium vapor pressure R above it or the solubility of solids. So, for example, over drops of liquid R higher than saturation pressure. pair ps over a flat liquid surface at the same temperature T. Respectively from fine particles in the environment is higher than the solubility c s flat surface of the same substance. These changes are described Kelvin equation, obtained from the condition of equality of chemical. potentials in adjacent phases in the state of thermodynamic. equilibrium:
where V- molar volume of a liquid or solid. For spherical particles g in abs. greater than their radius. Relegation or promotion R And from depends, in accordance with (4), on the sign of r (r>0 for convex, and r<0 для вогнутых поверхностей). Так, в отличие от рассмотренного выше случая давление пара в пузырьке или над поверхностью вогнутого мениска понижено: p

Equation (4) determines the direction of matter (from large values R And from to smaller) in the process of transition of the system to the state of thermodynamic. balance. This leads, in particular, to the fact that large droplets (or particles) grow due to the evaporation (dissolution) of smaller ones, and uneven surfaces (under the condition of a constant interfacial tension) are smoothed out due to the evaporation (dissolution) of protrusions and filling of depressions. Significant differences in pressure and solubility take place only at sufficiently small r (for water, for example, at |r|)