Ferric chloride color 3. Ferric chloride (iron (iii) chloride)
Iron(II) chloride
Chemical properties
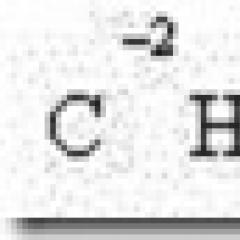
Ferric Chloride Formula: FeCl2 .
This compound is a medium salt of hydrochloric acid and iron. The substance is colorless crystals that oxidize and turn yellow in air. Melting point = 677 degrees Celsius. The product is highly soluble in water acetone And ethyl alcohol , insoluble in diethyl ether . Molar mass of a substance = 126.7 grams per mole.
Ferric chloride 2 tends to crystallize from aqueous solutions. Also, the substance in dry form, when heated in the presence of air, is easily oxidized to ferric chloride 3 , which has slightly different chemical properties.
Obtaining Ferric Chloride
The compound can be obtained by dissolving iron metal in hydrochloric acid , for example when boarding a steel product. Also in the laboratory, the substance can be synthesized from trivalent chloride , adding to it sulfur oxide and water or potassium iodide.
Ferric Chloride Reactions
The substance is used to obtain other chemical compounds, it is used in jewelry, in medicine, iron preparations are taken for treatment. iron deficiency anemia .
The drug is usually sold as an aqueous solution for oral administration.
pharmachologic effect
Antianemic.
Pharmacodynamics and pharmacokinetics
Thanks to this compound, it is possible to increase the level of iron in the body, stimulate the formation processes, and regulate various vital redox reactions.
With the passage of the course of the drug, the clinical symptoms are eliminated. anemia and improvement of laboratory parameters.
Usually the substance is enclosed in a plastic tradumet matrix , which provides a slow release of the active ingredient. After entering the gastrointestinal tract, the drug begins to be absorbed into the systemic circulation in the small intestine and duodenum. For processes liberalization affects the presence of water, the spent matrix is excreted from the body with feces, iron is absorbed and undergoes active metabolism. The more pronounced anemia, the better the drug is absorbed.
Indications for use
The drug is used for the prevention and treatment of iron deficiency conditions, including anemia . The drug is especially in demand in the treatment of children and adolescents, pregnant and lactating women, vegetarians and the elderly.
Contraindications
The tool is contraindicated:
- when on the active substance or auxiliary elements;
- patients with hemolytic anemia , hemosiderosis , hemochromatosis , aplastic anemia , thalassemia ;
- with violations of the processes of iron absorption ( lead anemia, sideroachrestic or pernicious anemia );
- allergy sufferers.
Side effects
During the course of treatment with this remedy, you may experience:
- discomfort in the gastrointestinal tract, a feeling of heaviness,;
- darkening of tooth enamel.
Iron Chloride, instructions for use (Method and dosage)
Ferric Chloride solution is taken orally. It can be diluted with juice or water. To avoid darkening of the teeth, it is recommended to drink the medicine through a straw.
As a prophylaxis, as a rule, 2 mg per kg of body weight per day is prescribed. The daily dosage is divided into 3-4 doses.
For treatment anemia use 4-6 mg per kg of body weight per day, the frequency of administration is the same.
For children from 1 to 12 years old, the daily dosage is up to 45 mg.
At the age of 12 years, 45 or more mg of medication per day is prescribed.
The duration of treatment is 60 days. Sometimes it is advisable to extend the treatment up to 3 months.
The maximum daily dosage of the drug is 200 mg.
Overdose
In case of an overdose, pain in the abdomen, vomiting, drowsiness, nausea, general weakness and pallor are observed, may develop shock or .
The victim needs to wash the stomach, carry out symptomatic treatment, the reception is indicated deferoxamine .
Interaction
Tetracyclines slow down the absorption of the drug. In this case, the drug reduces the absorption tetracyclines .
special instructions
To reduce the likelihood of side effects, the drug is recommended to be taken during or after a meal.
If the patient has a problem during treatment, then you need to reduce the single dose, but increase the frequency of administration.
During drug therapy, the stool may turn black, this is due to the fact that excess iron is excreted in the feces.
Preparations containing (Analogues)
Coincidence in the ATX code of the 4th level:Ferric chloride is contained in the preparation and aloe syrup with iron .
Attention! I myself have not tried that method, I just read about it in some book!
For the manufacture of ferric chloride, you need to take iron sawdust or thin plates and fill them with a solution of hydrochloric acid (HCl).
Sawdust is left for several days in an open container. After a few days, the solution will turn green.
After that, the resulting solution is drained and after a while it is ready for "work"!
P.S. On July 13, 2007, we received a letter from the respected Vladimir Syrov, in which he wrote the following:
For decades, a story about the possibility of making ferric chloride at home has been wandering around amateur radio literature. Here and on this site there is such (see above).
An unknown author honestly says "I myself have not tried this method." But, apparently, NONE of those who have ever written about it have tried this method !!! And your obedient servant tried it in the 90s, and the results are such that it is better not to even try to do it.
Iron can be either trivalent or divalent. When combined with chlorine, two formulas are obtained - "ferrum chlorine two" and "ferrum chlorine three". The first is green crystals, the second is yellow-brown. Only ferric chloride is suitable for etching copper printed circuit boards, "ferrum chlorine two" does not work - it has been established by experience. Or at least not working well. And with the described artisanal method (pouring iron filings with hydrochloric acid), according to some laws of chemistry, it is precisely "ferrum chlorine two" that is obtained. In some more detailed publications on this topic, this fact seems to be taken into account - they write something like "if you get a greenish
solution - let it stand in the open air so that it becomes yellowish-brown. "It has been verified by experience - it does not work! It stood for weeks and months ... Some insignificant part of ferrous iron is oxidized to ferric, but no more.
I tried to heat the solution, evaporate it, dry it and leave greenish crystals in the air .... To further oxidize by passing oxygen first through the solution, and then chlorine .... Everything is useless! I almost got poisoned myself and did not poison those around me, but I never got a practically significant result, a noticeable yield of "ferrum chlorine three"!
Please pay attention to the fact that we are dealing with poisons here! Hydrochloric acid is a solution of ash-chlorine gas in water. It "gases", that is, "ash-chlorine" evaporates from it. This gas, combining with water on the mucous membranes of the respiratory organs (nose, mouth, trachea and bronchi, lungs) - turns into the same hydrochloric acid! Chlorine, which I managed to get in sufficient quantities, is generally a specific poison. It is worth thinking about the fact that health is more expensive! At present, in any large city there is no problem to buy ferric chloride somewhere on the radio market and not suffer from its manufacture. As it turned out, in industry, chlorine (not chloride!) iron is obtained in a completely different way - by burning iron in an atmosphere of chlorine. It goes without saying that this method is hardly feasible at home.
Even in the presence of ready-made ferric chloride, I would advise you to be careful - to poison somewhere under air draft, on a balcony, somewhere in a garage .... To protect the health of not only your own, but also those of your immediate family. Not to mention lead, which is part of the tin-lead solder. Very small amounts of vapor
lead, getting into the body, causes chronic poisoning, various diseases, including tooth decay .... It is not for nothing that there are very strict instructions for the installation of exhaust ventilation in production. But at home, in everyday life, radio amateurs very often neglect this, but in vain. In fact, this lead is quite enough
a little. Only the consequences do not come immediately ... And there is little good in chlorides either ...
So the author of the publication (quoting someone) writes: "after a while the solution will turn green." It will be ferrous chloride, and not what should have been obtained. And about the fact that "after some time" it will still be ready for work .... Alas. If you don't believe me, try it yourself! And only then can you write a recipe when it
personally verified by experience. It is not worth writing from other people's words.
Qualitative reactions for iron (III)
Ions of iron (III ) in solution can be determined using qualitative reactions. Let's go through some of them. Take for the experiment a solution of iron chloride ( III).
1. III) - reaction with alkali.
If the solution contains iron ions ( III ), iron hydroxide is formed ( III ) Fe(OH) 3 . The base is insoluble in water and brown in color. (iron hydroxide ( II ) Fe(OH) 2 . - also insoluble, but grey-green in color). A brown precipitate indicates the presence of iron ions in the initial solution ( III).
FeCl 3 + 3 NaOH = Fe(OH) 3 ↓+ 3 NaCl
2. Qualitative reaction to iron ion ( III ) - reaction with yellow blood salt.
The yellow blood salt is hexacyanoferrate potassiumK 4 [ Fe( CN) 6]. (For the determination of iron (II) use red blood saltK 3 [ Fe( CN) 6 ]). To a portion of a solution of iron chloride, add a solution of yellow blood salt. The blue precipitate of Prussian blue* indicates the presence of ferric ions in the initial solution.
3 TO 4 +4 FeCl 3 = K Fe ) ↓ + 12 KCl
3. Qualitative reaction to iron ion ( III ) - reaction with potassium thiocyanate.
First, we dilute the test solution - otherwise we will not see the expected color. In the presence of an iron ion (III) when potassium thiocyanate is added, a red substance is formed. It is iron thiocyanateIII). Rhodanide from the Greek "rodeos" - red.
FeCl 3 + 3 KCNS= Fe( CNS) 3 + 3 KCl
Prussian blue was obtained by accident at the beginning of the 18th century in Berlin by the dyemaker Diesbach. Disbach bought an unusual potash (potassium carbonate) from a merchant: a solution of this potash turned blue when iron salts were added. When checking the potash, it turned out that it was calcined with bovine blood. The dye turned out to be suitable for fabrics: bright, stable and inexpensive. Soon the recipe for obtaining paint became known: potash was fused with dried animal blood and iron filings. By leaching such an alloy, yellow blood salt was obtained. Now Prussian blue is used to obtain printing ink and tint polymers. .
Equipment: flasks, pipette.
Safety . Observe the rules for handling alkali solutions and solutions hexacyanoferrates. Avoid contact of solutions of hexacyanoferrates with concentrated acids.
Statement of experience – Elena Makhinenko, text– Ph.D. Pavel Bespalov.
Ferric chloride- the average salt of ferric iron and hydrochloric acid. In appearance, this chemical raw material is a soft crystalline mass of a rusty-brownish-black color. Its boiling point is 319°C, melting point is 309°C. Ferric chloride is formed by heating iron with chlorine. It can also be obtained as a by-product in the production of titanium chloride TiCl4 and aluminum chloride AlCl3. Another way to obtain ferric chloride is hot chlorination or oxidation of the FeCl2 solution, followed by evaporation of the FeCl3 solution.
The scope of ferric chloride is quite wide. It is used as coagulant for water purification, as a catalyst in organic synthesis, as a mordant in the process of dyeing fabrics, as well as for the preparation of iron pigments and other iron salts. Another solution of ferric chloride is used for etching printed circuit boards.
Ferric chloride is widely used as a coagulant in the process of industrial and municipal wastewater treatment. Compared with other coagulants, in particular with aluminum sulfate, this chemical product has an important advantage - ferric chloride endowed with a high rate of deposition of various impurities. As a result of hydrolysis, ferric chloride forms a sparingly soluble iron hydroxide. In the process of its formation, various organic and inorganic impurities are captured, forming loose flakes, which are easily removed from the treated effluents. Such flakes, with a density of 1001–1100 g/l and a size of 0.5–3.0 mm, have a rather large surface with excellent sorption activity. In the process of their formation, the structure includes suspended substances (large microorganisms, plankton cells, silt, plant remains), colloidal particles, as well as part of the pollution ions associated on the surface of these particles. With the help of this product, the process of sedimentation of sludge proceeds much faster and deeper. Another advantage of ferric chloride is its beneficial effect on the biochemical decomposition of sludge. For high-quality wastewater treatment, 30 g of ferric chloride is required per cubic meter. Water purification with ferric chloride reduces the content of soluble impurities up to 25 percent, and insoluble impurities up to 95 percent. During the treatment of industrial and municipal wastewater, toxic compounds and microorganisms are destroyed by sodium hypochlorite.
Due to its pronounced acidic properties, iron chloride is used as a catalyst in organic synthesis processes, in the production of heat-resistant resins and in the oxidation of petroleum bitumen. Ferric chloride is an energetic chlorinating agent, so it is used for the selective extraction of certain components of ores. In particular, this chemical feedstock is required in aromatic hydrocarbons for the electrophilic substitution reaction. The use of aqueous solutions of ferric chloride is also well known. Possessing fairly mild etching properties, they are used in the electronics and instrumentation industries for etching printed circuit boards, metal parts, and copper foil. Applies ferric chloride and in construction. It is used as an additive to Portland cement to speed up the setting process. The addition of ferric chloride significantly increases the strength of concrete. This product is also used in other areas of human life, in particular:
with its help, natural waters in water treatment systems are clarified;
oil is removed from the effluents of fat-and-oil plants;
it is used in the treatment of wastewater from leather and fur enterprises from chromium compounds;
to soften domestic and drinking water;
as well as in organochlorine synthesis
Ferric chloride (III) in the form of a solution can be prepared in the laboratory or at home. You will need heat-resistant non-metallic utensils and clean hot or distilled). After dissolution and settling, a dark brown liquid is obtained. There are a number of features in the preparation of a ferric chloride solution that you should learn about before you start working with it.
Ferric chloride
Anhydrous ferric chloride, produced by the chemical industry - FeCl 3 - dark brown crystals with shades of red, purple, dark green. Molar mass - 162.21 g / mol. The substance melts at a temperature of 307.5 ° C, at 500 ° C it begins to decompose. A sample of anhydrous salt dissolves in 100 g of water:
- 74.4 g (0°C);
- 99 g (25 °C);
- 315 g (50 °C);
- 536 g (100 °C).
Anhydrous (III) - a very hygroscopic substance, quickly attracts moisture from the environment. In air, it interacts with water, turning into yellow crystals of FeCl 3 + 6H 2 O hexahydrate. The mass fraction of anhydrous ferric chloride in a substance purchased in a commercial network reaches 95%. There is a small amount of ferric chloride FeCl 2 and insoluble impurities. The trade name is Ferric Chloride. The substance is fire and explosion-proof, but its solution has a corrosive effect on metal objects.
Iron(III) chloride hexahydrate

In addition to anhydrous, the industry produces crystalline hydrate, in which the mass fraction of ferric chloride (III) is 60%. The substance is a yellow-brown crystalline mass or loose pieces of the same shade. An important distinguishing feature of ferrous and ferric ions is color. The oxidation state of Fe 2+ is characterized by a greenish tint, the hexahydrate iron chloride hydrate is a bluish-green substance. In the oxidation state of Fe 3+ ions acquire a color from yellow to brown. For a qualitative determination, reagents act on a solution of ferric chloride:
- NaOH (a brown precipitate of Fe (OH) 3 appears);
- K 4 (a blue precipitate of KFe appears);
- KCNS, NaCNS (red iron thiocyanate Fe(CNS) 3 is formed).
How to dilute ferric chloride

Iron (III) chloride in the form of a brown or red solution can be found in the commercial network, prepared in the laboratory or at home. In the latter case, you will definitely need heat-resistant non-metallic dishes (glass, plastic, ceramic). Water for dissolving salt can be taken from the tap. Safer - boiled or distilled. Water heated to 50-70 ° C is placed in a container, and then the substance is poured in small portions. The proportions of ferric chloride and water are 1:3. If you prepare a solution from crystalline hydrate, then less water will be required, because it is contained in crystalline hydrate (40% by weight). The substance is added to the solution little by little, each portion is about 5-10 g. It is not recommended to immediately pour the entire sample due to the rapid nature of the hydration reaction. Do not use metal utensils (spoons, spatulas). Salt must be completely dissolved in warm water, for which the crystals must be mixed well with the liquid. The process is accelerated by the addition of hydrochloric acid (1/10 of the mass of the crystals). After settling for several hours, a precipitate may appear at the bottom due to the presence in the sample and the formation of iron hydroxide during the reaction. The dark brown prepared solution should be filtered and stored in a tightly closed plastic container at a moderate temperature and out of direct sunlight.
The use of ferric chloride in industry and public utilities. domestic use
Iron salts find applications in many fields. Trivalent metal chloride is used for water treatment, metals and paint fixing. The substance is used in industrial organic synthesis (catalyst, oxidizer). The coagulating properties of the Fe 3+ ion are especially valued in the treatment of municipal and industrial wastewater. Under the action of ferric chloride, small insoluble particles of impurities stick together and precipitate. Also, there is a binding of a part of soluble contaminants, which are removed at the treatment plant. Crystal hydrate and anhydrous salt FeCl 3 are used in the etching of metal printing plates. A substance is added to concrete to strengthen its strength.
Chemical phenomena during etching of boards. Security measures

A popular chemical for PCB etching is ferric chloride. A solution for these purposes is prepared from 0.150 kg of salt and 0.200 l of warm water. It contains Fe 3+, Cl - ions, and upon hydrolysis, a brown compound is formed - ferric hydroxide. The process goes according to the scheme: FeCl 3 + 3HOH ↔ Fe (OH) 3 + 3Cl - + 3H +. The disadvantage of this method is the contamination of the board with reaction by-products, which make further etching difficult. Salt itself is a non-volatile substance, but in the process of interaction with water it releases caustic fumes. Work must be carried out outdoors or in a well-ventilated room. Contact with the solution on the skin and mucous membranes leads to irritation and can cause dermatitis. Personal protective equipment (goggles, gloves) should be used. In case of contact with a caustic solution, wash the skin with plenty of water.