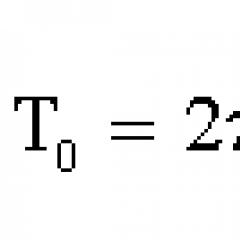Presentation on the topic: Basic provisions of the ICT. Presentation on physics on the topic “basic principles of molecular kinetic theory” Experiments proving this position
Topic: Basic provisions of the ICT
New topic questions:
1. MKT
2. Atom
3. Molecule
4. Basic provisions of the ICT
5. Diffusion
6. Brownian motion
7. Relative molecular weight
8. Amount of substance
9. 1 mole
10. Avogadro's number
11. Molar mass
12. Concentration of molecules

MKT
A) Molecular kinetic theory
B) Theory of thermal processes explaining the properties of macroscopic bodies
(Lomonosov M.V.)

Atom
A) The smallest indivisible particle
B) Discovered by Democritus
B) An atom consists of kernels and the surrounding electron cloud.
D) The nucleus of an atom consists of protons and neutrons, and the cloud surrounding it consists of electrons

Molecule
A) Electrically neutral particle
B) Has the basic chemical properties of a substance
B) Consists of atoms connected to each other by chemical bonds

Basic provisions of the ICT
1) All substances consist of particles (atoms and molecules)
2) Particles are in continuous motion
3) Particles interact with each other, i.e. between them there are attractive and repulsive forces

I. All substances are made up of particles
Experiments proving this position:
1. Mechanical crushing
2. Dissolution of a substance
3. Compression and stretching of bodies
Particles
molecules
atoms
electrons
core
protons
neutrons

PARTICLES MOVE CONTINUALLY AND CHAOTICALLY
EXPERIMENTS PROOF THIS POSITION:
- A) DIFFUSION
- B) BROWNIAN MOTION
- C) THE TENDENCY OF GAS TO OCCUPY THE ENTIRE VOLUME

Diffusion
A) Penetration of molecules of one substance into the intermolecular spaces of another substance
B) Diffusion depends on temperature

BROWNIAN MOTION
THERMAL MOTION OF PARTICLES SUSPENDED IN A LIQUID OR GAS OCCURS CHAOTICALLY AND UNLIMITED LONG TIMES

PARTICLES INTERACTING WITH EACH OTHER ARE ATTRACTED AND REPULED
Experiments confirming this position:
- Gluing
- Wetting

Relative molecular weight (RMM)
The ratio of the mass of a molecule to one-twelfth (1/12) of the mass of a carbon atom
- M r – OMM
- m 0 - mass of one molecule
- m 0c – mass of a carbon atom

QUANTITY OF SUBSTANCE
A value showing how many times the number of molecules in a substance is greater than the number of molecules in 12 grams of carbon
ν – amount of substance (mol)
N – number of molecules in a substance
N A – Avogadro's number
M – molar mass (kg/mol)

1 mole
A) The amount of a substance that contains the same number of molecules as are contained in 12 grams of carbon
B) Unit of measurement of the amount of substance

AVOGADRO'S NUMBER
A number showing how many molecules are contained in 1 mole of a substance
N A = 6,02 · 10 23 mole -1

Molar mass
The mass of a substance taken in the amount of one mole
M – molar mass (kg/mol)
m 0 – mass of one molecule

CONCENTRATION
A number showing how many particles are contained in a unit volume of a substance
n – concentration (m -3 )
V – volume (m 3 )
N – number of molecules

Control
1. What physical phenomenon is it based on? process of salting vegetables, fish, meat? In which case is the process faster?
If Is the brine cold or hot?
- Why does syrup become sweeter over time? taste of fruit?
- Why are sugar and other porous products Can't be stored near odorous substances?

- The smell of a birch broom spreads faster in a hot bath than in a cool room. Why?
- What do you think could happen as a result of a nuclear war?
- How can you explain the disappearance of smoke in the air?

1. OBJECTIVES
- 1. Find the mass of a water molecule if its molecular mass is 18 g/mol.
( m 0 =0,003*10 -23 kg)
- 2. How many molecules are there in 10 grams of aluminum? M ( Al) = 27 g/mol.
( N =2,2* 10 23 )
2. Testing
on the topic “Basic provisions of the ILC”

- § 4.1, 4.2, summary
- Task:
- What amount of substance is contained in 15 grams of copper if its molar mass is 64 g/mol?

The relative molecular (or atomic) mass of a substance M r is the ratio of the mass of a molecule (or atom) m 0 of a given substance to 1/12 of the mass of a carbon atom m 0C M r (H 2 O) = 2 1 + 16 = 18 (a.u. .m.) Let's calculate M r of water H 2 O (for this we will use the periodic table) Mendeleev Atomic mass unit (a.m.u.) 1.66 kg

One mole is the amount of a substance that contains the same number of molecules or atoms as there are atoms in carbon weighing 12 g. 1 mole of any substance contains the same number of atoms or molecules. This number of atoms is denoted N A and is called Avogadro’s constant in honor of the Italian scientist (19th century). N A = 6·10 23 mol -1






4. Thus, the gas pressure on the wall: P = = _______ This equation, first derived by the German physicist R. Clausius, is called the basic equation of the molecular kinetic theory of an ideal gas. It establishes a connection between microscopic parameters and macroscopic (measurable) quantities.







Macroscopic parameters include: ___ (________), ___ (_________) Thermal equilibrium is ... Temperature characterizes the degree of ... Temperature is measured using ... Page The Celsius scale (C) is determined by two points: ...

Page Experience: Conclusions: At 0C: At 100C:



Task. Given: Solution: Answer: pcs.



Page Experimental confirmation of the formula: Stern's experiment (1920)



Poll following the lesson: 1. How will the root mean square speed of molecules change when the temperature increases by 4 times? 2. Which molecules in the atmosphere move faster: nitrogen or oxygen molecules? Why? 3. What is absolute zero on the Celsius scale? 4. How are the volume, pressure and number of molecules of various gases related at thermal equilibrium?


Corollary: - Clapeyron equation R – universal gas constant Problem, 928











Physical dictation Option 1 Option 2



Dynamic equilibrium is a state in which _______ Saturated steam - steam, _______ The concentration of ________ saturated _____ at a constant ________ does not depend on its ____ Since p=nkT, the pressure of ________ steam _____________ on the volume it occupies. Saturated vapor pressure (p vp) is the vapor pressure at which ______ is in equilibrium with its _________.

Critical temperature is the maximum _____ at which _____ will turn into __ P n.a. at 100C equals Pa (760 mmHg) Mixture pressure: Components




Evaporation. Condensation. Boiling. Vaporization is the phenomenon of turning a liquid into vapor. I. - vaporization from the surface of a liquid, accompanied by the absorption of energy. Depends on: Temperature (tC - I.) Surface area of the liquid Type of liquid K. - vaporization throughout the entire volume of the liquid at a certain tC (boiling point). During boiling, the temperature does not change! (t=const)

During evaporation, only fast molecules that are able to overcome the attraction of other molecules fly out, and the temperature decreases (tC). boiling point depends on the ambient pressure and the presence of impurities. Inside the bubble is saturated steam, i.e. pressure Psteam = Water. t P steam V bubble Fa floats up Examples: “Blowing on tea”, “it’s cold when wet.” Examples: “Salt is added after boiling,” “in the mountains, water boils at 80 C.”


§Air humidity Why know about it? Books Fabrics Products Electrical appliances, etc. Air humidity is the content of water vapor in the atmosphere. In 1 year, billions of tons of water evaporate from the Earth's surface! Partial pressure of water P is the pressure produced by water vapor in the total atmospheric pressure.

Absolute humidity - the amount of water vapor per unit volume (water vapor density) Relative humidity - the ratio of absolute. humidity ρ to the density ρ 0 of saturated steam at a given temperature, expressed in %. P – partial pressure P 0 – saturated vapor pressure. P 0 and ρ 0 are found from the table




"Mono" - one. A monocrystal is a solid body whose particles form a single crystal lattice. An example of single crystals is the widespread hexagonal quartz crystals. Crystals of diamond, tourmaline, etc. are less common. They all have the characteristic shape of polyhedra h ttp:// /…

Anisotropy is the dependence of the physical properties of a substance on the direction. There are many particles in the A plane, they are located close to each other and there is a strong interaction. In plane B there are fewer particles, the distance between them is greater; the particles of the plane interact with each other weakly

Graphite and diamond are made of carbon...

Sapphire (corundum) contains impurities of titanium and iron. Microcircuits are formed on sapphires.

Second only to diamond among natural minerals, sapphire has an exceptionally high hardness - 9 points on the hardness scale out of a possible 10. Using sapphire powders you can drill, polish, sharpen stone and metal

Diamond is the hardest creation of nature. About 80% of all mined diamonds are used in industry.

Due to their hardness, diamonds are used for processing super-hard materials, exploration and mining. Before 1430, diamonds were not used as jewelry. The trendsetter for them was the Frenchwoman Agnes Sorel. ...

Among precious stones, ruby ranks second in strength after diamond. Some examples of ruby, distinguished by their particular purity of color, are valued even more than diamonds of the same weight...

Due to its high hardness, ruby is widely used in industry. Using ruby, you can produce an intense red beam of light millions of times brighter than the sun. Ruby lasers are used in scientific research to probe the atmosphere...

Emerald crystal Color depends on the content of chromium oxide ... ...

Deposits of emeralds are few in number. In the ancient world, mining was carried out only in Egypt. One of the best in our time is considered to be the mines in the Copi Mountains in the mountains of Colombia, which the Spanish conquerors captured in the 16th century. ...

A polycrystal is a solid body consisting of randomly oriented single crystals... In nature, randomly fused single crystals are more common. Examples of polycrystals are: rock salt (Fig. 1), quartz (Fig. 2), sugar, ice, iron, copper.

Among the bodies that are generally considered solid because they retain their shape and volume and have strength, there are also those that differ significantly from crystals. The particles in them are arranged in the same disorder as in a liquid. Amorphous bodies include glass, resin, rosin, and plastics. ...

CrystalsAmorphous bodies Particles are arranged in an orderly, strictly defined manner. There is no order in the arrangement of particles. Particles make “jumps” Melting at a certain temperature Do not have a specific melting point AnisotropicIsotropic Have a characteristic polyhedron shape Do not have the correct cut


Liquid crystals are substances that simultaneously have the properties of both liquids (fluidity) and crystals (anisotropy). In structure, they are liquids similar to jelly, consisting of elongated molecules, ordered in a certain way throughout the entire volume of this liquid. The external state of liquid crystals when heated can change from solid to liquid crystalline and completely transform into liquid form with a further increase in temperature.

Application of liquid crystals By selecting the composition of a liquid crystal substance, indicators are created for different temperature ranges and for various designs. For example, a liquid crystal indicator on a patient’s skin quickly diagnoses hidden inflammation and even a tumor. Liquid crystals are used to detect vapors of harmful chemical compounds and gamma and ultraviolet radiation hazardous to human health. Pressure meters and ultrasound detectors have been created based on liquid crystals. But the most promising area of application of liquid crystalline substances is information technology. Currently, color LCD screens are used in cell phones, monitors and televisions. They have small thickness, low power consumption, high resolution and brightness. ...

The world's first mineralogical reserve was created on the initiative of V. I. Vernadsky, N. M. Fedorovsky and A. E. Fersman in the Urals (in the Ilmen Mountains, near the city of Miass) in the first years of Soviet power...

The area of the reserve is 303.7 km². The length of the Ilmen ridge from north to south is 41 km. The highest peak is Mount Ilmentau (747.3 m) The hydrological network of the Reserve makes up 9% of the territory There are 30 lakes in the reserve The deepest lake Big Kisegach 34 m The longest river B. Cheremshanka 9.8 km Minerals, rocks Minerals 264 Discovered for the first time in the world in Ilmeny 16 Rocks over 70 Mines over 400

Russia, year 1842, October 8. At the Tsarevo-Aleksandrovsky mine near the city of Miass, in the Southern Urals, a gold nugget weighing 36 kg 16 g was found. Nowadays the “Big Triangle” - this is the name of the unique specimen - can be seen in the Diamond Fund of the Moscow Kremlin. It is considered the largest surviving one in the world. How many degrees will it heat up if it receives J of heat? The specific heat capacity of gold is 0.13 kJ/(kg K). Answer: 4 °C. ...

In 1813, on one of the tributaries of the Ural River Iset, where poor gold-bearing quartz veins were being mined, a young girl, Katya Bogdanova, found a large nugget of platinum and brought it to the clerk. What is the mass of a platinum nugget found in the Urals in 1904 if it would take J of energy to heat it by 20 °C? The specific heat capacity of platinum is 0.14 kJ/(kg * K). Answer: 8.395 kg.

1.What three types are solids divided into according to the nature of the arrangement of particles? What determines whether solids belong to one of these types? 2.What is the difference between mono- and polycrystals? 3.What bodies are classified as amorphous? 4.Wood is anisotropic. Is it a crystalline body? 5.Would the profession of glassblowing arise if glass were a crystalline body rather than an amorphous one?

1. A glass cube and a cube cut from a quartz single crystal are placed in hot water. Will the cubes keep their shape? 2. A cube cut from a single crystal can turn into a parallelepiped. Why is this possible? 3. You can drop an iron basin ten thousand times, but you cannot drop a porcelain vase even once. After all, ten thousand times you need ten thousand vases. Why? ...





Reason: the impacts of liquid molecules on a particle do not compensate each other. The nature of the movement depends on the type of liquid, the size and shape of the particles, and temperature. Brownian motion is the random continuous movement of tiny particles of a solid body suspended in a liquid or gas under the impacts of liquid or gas molecules. The nature of the movement depends on the type of liquid, the size and shape of the particles, and temperature. R. Brown 1827





The relative molecular (or atomic) mass of a substance M r is the ratio of the mass of a molecule (or atom) m 0 of a given substance to 1/12 of the mass of a carbon atom m 0C M r (H 2 O) = 2 1 + 16 = 18 a.u. m. Let's calculate M r of water H 2 O (for this we use the periodic table) Mendeleev Atomic mass unit (amu) 1.66 kg

One mole is the amount of a substance that contains the same number of molecules or atoms as there are atoms in carbon weighing 12 g. 1 mole of any substance contains the same number of atoms or molecules. This number of atoms is denoted N A and is called Avogadro’s constant in honor of the Italian scientist (19th century). N A = 6·10 23 mol -1

Basic provisions of the ICT. Sizes of molecules and atoms. Aggregate state of a substance.
(introduction to the topic “Thermodynamics”)

Basic principles of molecular kinetic theory
- All substances, without (exception), consist of particles.
The purpose of the ICT is explanation of the properties of macroscopic bodies and the laws of thermal processes based on the idea that all bodies consist of individual chaotically moving and interacting particles.





Sizes of molecules and atoms.
A drop of oil on the surface of a liquid will not spread over the entire free area; it forms a layer only one molecule thick - a “monomolecular layer”. If we take the volume of a drop of 1 mm 3, then it will spread to a maximum surface area of no more than 0.6 m 2, then we calculate:
d=0.001 cm 3: 6000 cm 2 ≈1.7×10⁻⁷cm.
The diameter of a water molecule is approximately 3 × 10⁻⁸ cm, then there are 3.7 × 10 22 molecules in 1 cm 3. The mass of the molecule will be 2.7 × 10⁻ 23 g.
- The mass of all molecules and atoms is compared to 1/12 the mass of a carbon atom.
- Relative molecular weight is determined by the formula:
M r = m m / m c/ 12
Where m is the mass of a molecule of a substance,
m c is the mass of a carbon atom.
- Relative atomic mass A r is the ratio of the mass of an atom to 1/12 of the mass of a carbon atom.

Formulas and definitions on the topic.
1 amu = mc / 12 = 1,66 × 10 -27 kg
The number of molecules in one mole of a substance is called Avogadro's constant: N a = 6.02 × 10 23 mol -1
Avogadro's law: equal volumes of different gases under the same conditions always contain the same number of particles.
The unit of quantity of substance ν is the mole.
Mol- is the amount of a substance that contains the same number of molecules (atoms) as there are in 12 g of carbon.
The mass of molecules of a substance can be determined as follows:
Molar mass M - This is the mass of 1 mole of a substance.
m m = M / N a; m m = m / N; m m = ρ / n
Mass of substance m = M× ν
m is the mass of the substance, N is the number of molecules,
SI unit of molar mass
n is the concentration of molecules.
kg/mol M = M r ×10 -3
The number of molecules of a substance can be determined as follows:
N = N a × ν; N = m / m m; N=n×V
The volume V M of one mole of a substance is determined by the formula:
V = M / ρ ρ – density of matter

State of matter
- Solid
- Liquid
- Gaseous
The most abundant substance on Earth is water. Let's consider it in all states and remember,
that the molecule itself is not
Changes.

Solid state of matter
Molecules are located at certain distances from each other,
making oscillatory
movement at position
balance. Interaction
there is a very strong connection between them,
Therefore, in a solid state, bodies retain their shape and volume.
(in the picture of an ice molecule
and photo of the ice city).

Liquid state of matter
Molecules are located extremely close to each other, which makes liquids practically
incompressible. Movement them
messy and they move
throughout the volume, this causes fluidity
substances in this state of aggregation.
The attraction between particles is weak,
therefore liquids easily spill over
portions. Consequently, liquids retain their volume but easily take the shape of the container.

Gaseous state of matter
The molecules are located far enough apart from each other, which allows them to scatter over long distances and
don't interact between
yourself. Therefore the gases are not
have a constant volume
they strive to fill all the space given to them. Consequently, gases do not have their own shape and volume.

Consolidation
1.Formulate the main provisions of the ICT.
2. Provide facts confirming the validity of these provisions.
3. Solve the problem: determine the molar mass of sugar
4. How many molecules are there in 210 g of nitrogen?
Students can be asked to solve problems at home.

1. All substances, without (exception), consist of particles.
All particles move randomly.
All particles of matter interact with each other.
2. Crushing, evaporation, dissolution, diffusion, Brownian motion, spreading of liquid, the appearance of elasticity, deformation, preservation of the shape and volume of solids, friction.
3. M r (C 12 H 22 O 11) = 12 A r (C) + 22 A r (H) + 11 A r (O)
M r = 12 12 + 22 + 11 16 = 342 (amu)
M = M r 10 -3 = 0.342 (kg/mol).
4. m(N 2) = 0.21 kg
N = m / m N 2 m N 2 = M / N a N = m N a /m