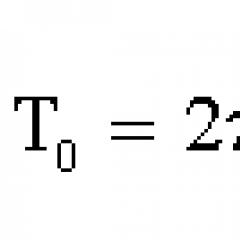X-rays. Presentation on physics "X-ray radiation" Germicidal lamps and irradiators
"X-Rays" Gulikyan Hamlet
Discovery of X-rays X-rays were discovered in 1895 by the German physicist Wilhelm Roentgen. Roentgen knew how to observe, he knew how to notice something new where many scientists before him had not discovered anything remarkable. This special gift helped him make a remarkable discovery. At the end of the 19th century, gas discharge at low pressure attracted the attention of physicists. Under these conditions, flows of very fast electrons were created in the gas-discharge tube. At that time they were called cathode rays. The nature of these rays has not yet been established with certainty. All that was known was that these rays originated at the cathode of the tube. Having started studying cathode rays, Roentgen soon noticed that the photographic plate near the discharge tube was overexposed even when it was wrapped in black paper.
Discovery of X-rays The scientist realized that when the discharge tube operates, some previously unknown, highly penetrating radiation appears. He called them X-rays. Subsequently, the term “X-rays” became firmly established behind this radiation. X-ray discovered that new radiation appeared in the place where the cathode rays (streams of fast electrons) collided with the glass wall of the tube. In this place the glass glowed with a greenish light.
Properties of X-rays The rays discovered by Roentgen acted on a photographic plate, caused ionization of the air, but were not noticeably reflected from any substances and did not undergo refraction. The electromagnetic field had no effect on the direction of their propagation.
Properties of X-rays The assumption immediately arose that X-rays are electromagnetic waves that are emitted when electrons are sharply decelerated. Unlike visible light and ultraviolet rays, X-rays have a much shorter wavelength. Their wavelength is shorter, the greater the energy of the electrons colliding with the obstacle.
X-Ray Diffraction If X-rays are electromagnetic waves, then they should exhibit diffraction, a phenomenon common to all types of waves. First, X-rays were passed through very narrow slits in lead plates, but nothing resembling diffraction could be detected. German physicist Max Laue suggested that the wavelength of X-rays was too short to detect diffraction of these waves by artificially created obstacles. After all, it is impossible to make slits 10-8 cm in size, since this is the size of the atoms themselves. What if the X-rays are about the same full length? Then the only option left is to use crystals. They are ordered structures in which the distances between individual atoms are equal in order of magnitude to the size of the atoms themselves, i.e. 10-8 cm. A crystal with its periodic structure is that natural device that should inevitably cause noticeable wave diffraction if the length they are close to the size of atoms.
Diffraction of X-rays And so a narrow beam of X-rays was directed at the crystal, behind which a photographic plate was located. The result was completely consistent with the most optimistic expectations. Along with the large central spot, which was produced by rays propagating in a straight line, regularly spaced small spots appeared around the central spot (Fig. 50). The appearance of these spots could only be explained by the diffraction of X-rays on the ordered structure of the crystal. The study of the diffraction pattern made it possible to determine the wavelength of the X-rays. It turned out to be smaller than the wavelength of ultraviolet radiation and in order of magnitude was equal to the size of an atom (10-8 cm).
Applications of X-rays X-rays have found many very important practical applications. In medicine, they are used to make the correct diagnosis of a disease, as well as to treat cancer. The applications of X-rays in scientific research are very extensive. From the diffraction pattern produced by X-rays when they pass through crystals, it is possible to establish the order of arrangement of atoms in space - the structure of the crystals. It turned out to be not very difficult to do this for inorganic crystalline substances. But with the help of X-ray diffraction analysis it is possible to decipher the structure of complex organic compounds, including proteins. In particular, the structure of the hemoglobin molecule, containing tens of thousands of atoms, was determined.
X-ray tube design Currently, very advanced devices called X-ray tubes have been developed to produce X-rays. Figure 51 shows a simplified diagram of an electron X-ray tube. Cathode 1 is a tungsten helix that emits electrons due to thermionic emission. Cylinder 3 focuses the flow of electrons, which then collide with the metal electrode (anode) 2. This produces x-rays. The voltage between the anode and cathode reaches several tens of kilovolts. A deep vacuum is created in the tube; the gas pressure in it does not exceed 10-5 mm Hg. Art.
Slide 1
X-RAYS Physics teacher Natalia Borisovna Trifoeva School No. 489, Moscow district of St. PetersburgSlide 2
 Discovery of X-rays At the end of the 19th century, gas discharge at low pressure attracted the attention of physicists. Under these conditions, flows of very fast electrons were created in the gas-discharge tube. At that time they were called cathode rays. The nature of these rays has not yet been established with certainty. All that was known was that these rays originated at the cathode of the tube. Roentgen Wilhelm (1845-1923) - German physicist who discovered short-wave electromagnetic radiation - X-rays - in 1895.
Discovery of X-rays At the end of the 19th century, gas discharge at low pressure attracted the attention of physicists. Under these conditions, flows of very fast electrons were created in the gas-discharge tube. At that time they were called cathode rays. The nature of these rays has not yet been established with certainty. All that was known was that these rays originated at the cathode of the tube. Roentgen Wilhelm (1845-1923) - German physicist who discovered short-wave electromagnetic radiation - X-rays - in 1895.
Slide 3
 Discovery of X-rays While studying cathode rays, Roentgen noticed that a photographic plate near the discharge tube was illuminated even when it was wrapped in black paper. After this, he was able to observe another phenomenon that really amazed him. A paper screen moistened with a solution of barium platinum oxide began to glow if it was wrapped around the discharge tube. Moreover, when Roentgen held his hand between the tube and the screen, dark shadows of the bones were visible on the screen against the background of the lighter outlines of the entire hand. The scientist realized that when the discharge tube was operating, some previously unknown, highly penetrating radiation was generated. He called them X-rays. Subsequently, the term “X-rays” became firmly established behind this radiation. X-ray discovered that new radiation appeared in the place where the cathode rays (streams of fast electrons) collided with the glass wall of the tube. In this place the glass glowed with a greenish light. Subsequent experiments showed that X-rays arise when fast electrons are slowed down by any obstacle, in particular metal electrodes.
Discovery of X-rays While studying cathode rays, Roentgen noticed that a photographic plate near the discharge tube was illuminated even when it was wrapped in black paper. After this, he was able to observe another phenomenon that really amazed him. A paper screen moistened with a solution of barium platinum oxide began to glow if it was wrapped around the discharge tube. Moreover, when Roentgen held his hand between the tube and the screen, dark shadows of the bones were visible on the screen against the background of the lighter outlines of the entire hand. The scientist realized that when the discharge tube was operating, some previously unknown, highly penetrating radiation was generated. He called them X-rays. Subsequently, the term “X-rays” became firmly established behind this radiation. X-ray discovered that new radiation appeared in the place where the cathode rays (streams of fast electrons) collided with the glass wall of the tube. In this place the glass glowed with a greenish light. Subsequent experiments showed that X-rays arise when fast electrons are slowed down by any obstacle, in particular metal electrodes.
Slide 4
 Properties of X-rays The rays discovered by Roentgen acted on a photographic plate, caused ionization of the air, but were not noticeably reflected from any substances and did not undergo refraction. The electromagnetic field had no effect on the direction of their propagation. The assumption immediately arose that X-rays are electromagnetic waves that are emitted when electrons are sharply decelerated. Unlike visible light and ultraviolet rays, X-rays have a much shorter wavelength. Their wavelength is shorter, the greater the energy of the electrons colliding with the obstacle. The high penetrating power of X-rays and their other features were associated precisely with the short wavelength. But this hypothesis needed evidence, and evidence was obtained 15 years after Roentgen’s death.
Properties of X-rays The rays discovered by Roentgen acted on a photographic plate, caused ionization of the air, but were not noticeably reflected from any substances and did not undergo refraction. The electromagnetic field had no effect on the direction of their propagation. The assumption immediately arose that X-rays are electromagnetic waves that are emitted when electrons are sharply decelerated. Unlike visible light and ultraviolet rays, X-rays have a much shorter wavelength. Their wavelength is shorter, the greater the energy of the electrons colliding with the obstacle. The high penetrating power of X-rays and their other features were associated precisely with the short wavelength. But this hypothesis needed evidence, and evidence was obtained 15 years after Roentgen’s death.
Slide 5
 X-Ray Diffraction If X-rays are electromagnetic waves, then they should exhibit diffraction, a phenomenon common to all types of waves. First, X-rays were passed through very narrow slits in lead plates, but nothing resembling diffraction could be detected. German physicist Max Laue suggested that the wavelength of X-rays was too short to detect diffraction of these waves by artificially created obstacles. After all, it is impossible to make slits 10-8 cm in size, since this is the size of the atoms themselves. What if X-rays have approximately the same wavelength? Then the only option left is to use crystals. They are ordered structures in which the distances between individual atoms are equal in order of magnitude to the size of the atoms themselves, i.e. 10-8 cm. A crystal with its periodic structure is that natural device that should inevitably cause noticeable wave diffraction if the length they are close to the size of atoms.
X-Ray Diffraction If X-rays are electromagnetic waves, then they should exhibit diffraction, a phenomenon common to all types of waves. First, X-rays were passed through very narrow slits in lead plates, but nothing resembling diffraction could be detected. German physicist Max Laue suggested that the wavelength of X-rays was too short to detect diffraction of these waves by artificially created obstacles. After all, it is impossible to make slits 10-8 cm in size, since this is the size of the atoms themselves. What if X-rays have approximately the same wavelength? Then the only option left is to use crystals. They are ordered structures in which the distances between individual atoms are equal in order of magnitude to the size of the atoms themselves, i.e. 10-8 cm. A crystal with its periodic structure is that natural device that should inevitably cause noticeable wave diffraction if the length they are close to the size of atoms.
Slide 6
 X-ray diffraction A narrow beam of X-rays was directed at a crystal behind which a photographic plate was located. The result was completely consistent with the most optimistic expectations. Along with the large central spot, which was produced by rays propagating in a straight line, regularly spaced small spots appeared around the central spot (Fig. 1). The appearance of these spots could only be explained by the diffraction of X-rays on the ordered structure of the crystal. The study of the diffraction pattern made it possible to determine the wavelength of the X-rays. It turned out to be smaller than the wavelength of ultraviolet radiation and in order of magnitude was equal to the size of an atom (10-8 cm). Fig.1
X-ray diffraction A narrow beam of X-rays was directed at a crystal behind which a photographic plate was located. The result was completely consistent with the most optimistic expectations. Along with the large central spot, which was produced by rays propagating in a straight line, regularly spaced small spots appeared around the central spot (Fig. 1). The appearance of these spots could only be explained by the diffraction of X-rays on the ordered structure of the crystal. The study of the diffraction pattern made it possible to determine the wavelength of the X-rays. It turned out to be smaller than the wavelength of ultraviolet radiation and in order of magnitude was equal to the size of an atom (10-8 cm). Fig.1
Slide 7
 Applications of X-rays X-rays have found many very important practical applications. In medicine, they are used to make the correct diagnosis of a disease, as well as to treat cancer. The applications of X-rays in scientific research are very extensive. From the diffraction pattern produced by X-rays when they pass through crystals, it is possible to establish the order of arrangement of atoms in space - the structure of the crystals. Using X-ray diffraction analysis, it is possible to decipher the structure of complex organic compounds, including proteins. In particular, the structure of the hemoglobin molecule, containing tens of thousands of atoms, was determined. These advances were made possible by the fact that the wavelength of X-rays is very short, which is why it was possible to “see” molecular structures. Among other applications of X-rays, we note X-ray flaw detection - a method for detecting cavities in castings, cracks in rails, checking the quality of welds, etc. X-ray flaw detection is based on a change in the absorption of X-rays in a product if there is a cavity or foreign inclusions in it.
Applications of X-rays X-rays have found many very important practical applications. In medicine, they are used to make the correct diagnosis of a disease, as well as to treat cancer. The applications of X-rays in scientific research are very extensive. From the diffraction pattern produced by X-rays when they pass through crystals, it is possible to establish the order of arrangement of atoms in space - the structure of the crystals. Using X-ray diffraction analysis, it is possible to decipher the structure of complex organic compounds, including proteins. In particular, the structure of the hemoglobin molecule, containing tens of thousands of atoms, was determined. These advances were made possible by the fact that the wavelength of X-rays is very short, which is why it was possible to “see” molecular structures. Among other applications of X-rays, we note X-ray flaw detection - a method for detecting cavities in castings, cracks in rails, checking the quality of welds, etc. X-ray flaw detection is based on a change in the absorption of X-rays in a product if there is a cavity or foreign inclusions in it.
Slide 8
 X-ray tube design Currently, very advanced devices called X-ray tubes have been developed to produce X-rays. In Fig. Figure 2 shows a simplified diagram of an electron X-ray tube. Cathode 1 is a tungsten helix that emits electrons due to thermionic emission. Cylinder 3 focuses the flow of electrons, which then collide with the metal electrode (anode) 2. This produces x-rays. The voltage between the anode and cathode reaches several tens of kilovolts. A deep vacuum is created in the tube; the gas pressure in it does not exceed 10-5 mm Hg. Art. In powerful X-ray tubes, the anode is cooled by running water, since a large amount of heat is released when electrons are decelerated. Only about 3% of the electron energy is converted into useful radiation. Fig.2
X-ray tube design Currently, very advanced devices called X-ray tubes have been developed to produce X-rays. In Fig. Figure 2 shows a simplified diagram of an electron X-ray tube. Cathode 1 is a tungsten helix that emits electrons due to thermionic emission. Cylinder 3 focuses the flow of electrons, which then collide with the metal electrode (anode) 2. This produces x-rays. The voltage between the anode and cathode reaches several tens of kilovolts. A deep vacuum is created in the tube; the gas pressure in it does not exceed 10-5 mm Hg. Art. In powerful X-ray tubes, the anode is cooled by running water, since a large amount of heat is released when electrons are decelerated. Only about 3% of the electron energy is converted into useful radiation. Fig.2
Description of the presentation by individual slides:
1 slide
Slide description:
2 slide
Slide description:
It was a rare person who did not go through the X-ray room. And pictures taken with X-rays are familiar to everyone. X-ray radiation was discovered by the German physicist W. Roentgen (1845–1923). His name is immortalized in several other physical terms associated with this radiation: the roentgen is the international unit of dose of ionizing radiation; a picture taken in an X-ray machine is called a radiograph; The field of radiological medicine that uses x-rays to diagnose and treat diseases is called radiology.
3 slide
Slide description:
Roentgen further established that the penetrating ability of the unknown rays he discovered, which he called X-rays, depends on the composition of the absorbing material. He also obtained an image of the bones of his own hand by placing it between a discharge tube with cathode rays and a screen coated with barium cyanoplatinite. Roentgen discovered radiation in 1895 while a professor of physics at the University of Würzburg. While conducting experiments with cathode rays, he noticed that a screen located near the vacuum tube, covered with crystalline barium cyanoplatinite, glowed brightly, although the tube itself was covered with black cardboard. This is how Roentgen himself first illuminated his hand in 1895.
4 slide
Slide description:
New rays appeared in the so-called discharge tube, where a stream of negatively charged particles fell, decelerating, onto the target. A little later it turned out that these particles were electrons. Roentgen himself, not knowing about the existence of the electron, could not explain the nature of the rays he discovered. Flow of electrons X-rays X-ray radiation, electromagnetic radiation invisible to the eye with a wavelength of 10-7 - 10-14 m. Emitted during the deceleration of fast electrons in a substance (bremsstrahlung spectrum) and during transitions of electrons in an atom from outer electron shells to inner ones (characteristic spectrum).
5 slide
Slide description:
Roentgen's discovery was followed by experiments by other researchers who discovered many new properties and applications of this radiation. A major contribution was made by M. Laue, W. Friedrich and P. Knipping, who demonstrated in 1912 the diffraction of X-ray radiation when passing through a crystal; W. Coolidge, who in 1913 invented a high-vacuum X-ray tube with a heated cathode; G. Moseley, who established in 1913 the relationship between the wavelength of radiation and the atomic number of an element; G. and L. Bragg, who received the Nobel Prize in 1915 for developing the fundamentals of X-ray structural analysis.
6 slide
Slide description:
Sources of X-ray radiation: X-ray tube, electron accelerators, lasers, solar corona, celestial bodies.
7 slide
Slide description:
Properties of X-ray radiation Has great penetrating power, Causes luminescence, Actively affects the cells of a living organism, Capable of causing gas ionization and the photoelectric effect, Interacts with atoms of the crystal lattice, Interference and diffraction on the crystal lattice is observed, Almost does not refract or reflect, Irradiation in high doses causes radiation sickness.
8 slide
Slide description:
X-ray radiation is invisible to the eye, so all observations with it are carried out using fluorescent screens or photographic films. X-ray receivers - photographic film, x-ray screen, etc. Penetrates through some opaque materials. It is used in medicine, flaw detection, spectral and structural analysis.
Slide 9
Slide description:
Like visible light, X-rays cause photographic film to turn black. This property is important for medicine, industry and scientific research. Passing through the object under study and then falling onto the photographic film, X-ray radiation depicts its internal structure on it. Since the penetrating power of X-ray radiation varies for different materials, parts of the object that are less transparent to it produce lighter areas in the photograph than those through which the radiation penetrates well. Thus, bone tissue is less transparent to x-rays than the tissue that makes up the skin and internal organs. Therefore, on an x-ray, the bones will appear as lighter areas and the fracture site, which is more transparent to radiation, can be detected quite easily. X-rays are also used in dentistry to detect caries and abscesses in the roots of teeth, and in industry to detect cracks in castings, plastics and rubbers.
10 slide
Slide description:
X-rays are used in chemistry to analyze compounds and in physics to study the structure of crystals. An X-ray beam passing through a chemical compound produces characteristic secondary radiation, the spectroscopic analysis of which allows the chemist to determine the composition of the compound. When falling on a crystalline substance, a beam of X-rays is scattered by the atoms of the crystal, giving a clear, regular picture of spots and stripes on a photographic plate, which makes it possible to establish the internal structure of the crystal. The use of X-rays in cancer treatment is based on the fact that it kills cancer cells. However, it can also have undesirable effects on normal cells. Therefore, extreme caution must be exercised when using X-rays in this manner. X-ray radiation is also used in art history and forensics.
11 slide
Slide description:
OBTAINING X-RAY RADIATION X-ray radiation occurs when electrons moving at high speeds interact with matter. When electrons collide with atoms of any substance, they quickly lose their kinetic energy. In this case, most of it turns into heat, and a small fraction, usually less than 1%, is converted into X-ray energy. This energy is released in the form of quanta - particles called photons, which have energy but whose rest mass is zero. X-ray photons differ in their energy, which is inversely proportional to their wavelength. The conventional method of producing X-rays produces a wide range of wavelengths, which is called the X-ray spectrum.
12 slide
Slide description:
If an electron collides with a relatively heavy nucleus, it is decelerated, and its kinetic energy is released in the form of an X-ray photon of approximately the same energy. If it flies past the nucleus, it will lose only part of its energy, and the rest will be transferred to other atoms that come across its path. Each act of energy loss leads to the emission of a photon with some energy. A continuous X-ray spectrum appears, the upper limit of which corresponds to the energy of the fastest electron. X-ray radiation can be obtained not only by electron bombardment, but also by irradiating a target with X-ray radiation from another source. In this case, however, most of the energy of the incident beam goes into the characteristic X-ray spectrum and a very small proportion of it falls into the continuous one. It is obvious that the beam of incident X-ray radiation must contain photons whose energy is sufficient to excite the characteristic lines of the bombarded element. The high percentage of energy per characteristic spectrum makes this method of excitation of X-ray radiation convenient for scientific research.
Slide 13
Slide description:
Another important use of X-rays is in astronomy. It is difficult to detect this radiation on Earth due to absorption in the atmosphere. But when the instruments began to be lifted on rockets and satellites, they recorded X-ray radiation from the Sun and stars. The main thing is that we managed to catch such rays from previously unknown celestial objects - pulsars. These are like X-ray beacons flashing to us from the distant expanses of space.
14 slide
Slide description:
1. Match. 1. V. Roentgen discovered new radiation while researching... 2. These rays appeared on... 3. The scientist observed... 4. V. Roentgen established that when a gas-discharge tube operates, A. appears at the anode of the gas-discharge tube. B. Glass where cathode rays hit it. The glow of a screen moistened with a solution of barium platinum oxide located near the tube. G. Cathode rays. D. Previously unknown radiation with high penetrating power. E. X-ray radiation (X-rays). 2. Match. 1. B. Roentgen discovered that new radiation arises on... 2. Subsequent experiments showed what cathode rays are. 3. It was discovered that X-rays arise from... A. Streams of very fast electrons. B. Cathode of the gas discharge tube. Braking of electrons by any obstacle. D. Previously unknown radiation with high penetrating power. D. Anode of the gas discharge tube. E. Acceleration of electrons by an electric field. The figure shows a diagram of an X-ray tube. establish a match. 1. Free electrons appear in the tube as a result of... 2. The acceleration of electrons when moving towards the anode occurs under the influence of... 3. A positive potential is applied to... 4. The voltage between the electrodes of the X-ray tube reaches... 5. To increase the electron mean free path, the gas pressure in the X-ray tube must be equal to the electric field. B. Thermionic emission. Anode. G. 104 V. D. Cathode. E. Very low. F. 103 V. 3. Low.
VPAKENORAVIDYTRLBHYU RADIATIONCHAVFRIETORGSHIINFRAREDOTYLNSHVRGJBZHULTRAVIOLETROKUAVFMONSHTRENTRENOVSKOESYANGR .

Types of radiation: infrared, ultraviolet, x-ray
Physics lesson in 11th grade
Teacher: Vlasova O.V.
NOU Secondary School No. 47 JSC Russian Railways
Ingol village, Krasnoyarsk Territory

Visible spectrum
400THz 800THz
760nm 380nm

History of the discovery of infrared radiation
English astronomer and physicist
William Herschel.

History of discovery
Beyond the visible red stripe, the temperature of the thermometer rises.

- Atoms and molecules of matter.
- All bodies at any temperature.

Infrared radiation sources
Sun.
Incandescent lamps.

Wave and frequency range of infrared radiation
- Wavelength
λ = 8*10 -7 – 2*10 -3 m.
- Frequency
υ= 3*10 11 – 4*10 14 Hz.

Properties of infrared radiation
- Invisible.
- Produces a chemical effect on photographic plates.
- Water and water vapor are not transparent.
- When absorbed by a substance, it heats it up.

Biological effect
At high temperatures it is dangerous for the eyes and can cause vision damage or blindness.
Means of protection:
special infrared glasses.

Infrared heater
Thermal imager
Thermogram

Applications of infrared radiation
In night vision devices:
- binoculars;
- glasses;
- sights for small arms;
- night photos and video cameras.

Thermal imager is a device for monitoring the temperature distribution of the surface under study.
Application of IR radiation
Thermogram - infrared image showing the distribution of temperature fields .

Infrared radiation in medicine
Thermograms are used in medicine to diagnose diseases.

Application of infrared radiation in thermal imagers
Monitoring the thermal state of objects.

Infrared radiation in construction
Checking the quality of building materials and insulation .


Applications of infrared radiation
Remote control.

The total length of fiber-optic communication lines is more than 52 thousand kilometers.

Application of infrared radiation on railways
Providing light to fiber optic communication systems using infrared lasers.

Used in railway transport
one-, two- and three-cable methods of organizing communication lines. Optical cables contain
4, 8 and 16 fibers.

Fiber – optical communication system
Simultaneous transmission
10 million telephone conversations and
1 million video signals.

Fiber – optical communication system
The fiber lifetime exceeds 25 years.


Application of infrared radiation on railways
Control of rolling stock from the transportation dispatch control center.


History of discovery
German physicist Johann Wilhelm Ritter.
English scientist
W. Wollaston.

UV sources
- Sun, stars.
- High temperature plasma.
- Solids with
temperature
above 1000 0 WITH.
- All bodies are heated
over 3000 0 WITH.
- Quartz lamps.
- Electric arc.

Wave and frequency range of ultraviolet radiation
- Wavelength
λ = 10 -8 – 4*10 -7 m.
- Frequency
υ= 8*10 14 – 3*10 15 Hz.

Properties of ultraviolet radiation
- Invisible.
- All properties of electromagnetic waves (reflection, interference, diffraction and others).
- Ionizes the air.
- Quartz is transparent, glass is not.

Biological effect
- Kills microorganisms.
- In small doses, it promotes the formation of vitamins D, growth and strengthening of the body.
- A tan.
- In large doses, it causes changes in cell development and metabolism, skin burns, and eye damage.
Methods of protection:
glass glasses and sunscreen.

Features of ultraviolet radiation
With an increase in altitude for every 1000 m
UV level
increases by 12%.

Application of Ultraviolet Radiation
Creation of luminous colors.
Currency detector.
A tan.
Making stamps.

in medicine
Germicidal lamps and irradiators.
Laser biomedicine.
Disinfection.
In cosmetology – solarium lamps.

in the food industry
Sterilization (disinfection) of water, air and various surfaces.

Application of Ultraviolet Radiation in Forensic Science
In devices for detecting traces of explosives.

in Printing
Production of seals and stamps.

To protect banknotes
- Protection of bank cards and banknotes from counterfeiting.
- Currency detector.



The service life of an incandescent lamp is no more than 1000 hours.
Luminous efficacy 10-100 lm/W.

Application ultraviolet radiation on the railway
LED lifespan
50000 hours
and more.
Luminous output exceeds
120 lm/W and constantly growing.

Application of ultraviolet radiation on railways
Emitter
with a small temperature shift along the wavelength and a long lifetime.


History of discovery
German physicist Wilhelm Roentgen.
Honored
Nobel Prize.

X-ray sources
- Free electrons moving with high acceleration.
- Electrons of the inner shells of atoms changing their states.
- Stars and galaxies.
- Radioactive decay of nuclei.
- Laser .
- X-ray tube.

Wave and frequency range of X-ray radiation
- Wavelength
λ = 10 -8 – 10 -12 m.
- Frequency
υ= 3 . 10 16 – 3 . 10 20 Hz.

Properties of X-rays
- Invisible.
- All properties of electromagnetic waves (reflection, interference, diffraction and others).
- Great penetrating power.
- Strong biological effect.
- High chemical activity.
- Causes some substances to glow - fluorescence.

Biological effect
- Ionizing.
- Causes radiation sickness, radiation burns and malignant tumors.


In medicine
Diagnostics
X-ray therapy



- Flaw detection.
- X-ray diffraction analysis.



ARE COMMON
- All electromagnetic waves are of the same physical nature.
- They occur when electrical charges move at an accelerated rate.
All electromagnetic waves have the following properties: interference, diffraction, reflection, polarization, refraction, absorption.
They propagate in a vacuum at a speed of 300,000 km/s.

PROPERTIES OF ELECTROMAGNETIC RADIATIONS
DIFFERENCES
As the frequency increases:
- Reducing the wavelength.
Increase in radiation energy.
Weaker absorption by the substance.
Increased penetrating power.
A stronger manifestation of quantum properties.
Increased harmful effects on living organisms.

Ultraviolet
radiation
radiation
Infrared
radiation
Radio waves
Gamma radiation
Fast moving
Discovery of X-rays. In 1894, when Roentgen was elected rector of the university, he began experimental studies of electric discharge in glass vacuum tubes. On the evening of November 8, 1895, Roentgen, as usual, was working in his laboratory, studying cathode rays. Around midnight, feeling tired, he got ready to leave. Looking around the laboratory, he turned off the light and was about to close the door, when he suddenly noticed some luminous spot in the darkness. It turns out that a screen made of barium bluehydride was glowing. Why is it glowing? The sun had long set, electric light could not cause a glow, the cathode tube was turned off, and in addition it was covered with a black cardboard cover. X-ray looked at the cathode tube again and reproached himself: it turns out that he forgot to turn it off. Having felt the switch, the scientist turned off the receiver. The glow of the screen also disappeared; turned on the handset again - and the glow appeared again. This means that the glow is caused by the cathode tube! But how? After all, the cathode rays are delayed by the cover, and the meter-long air gap between the tube and the screen is armor for them. Thus began the birth of the discovery.
Slide 5 from the presentation “X-ray physics” for physics lessons on the topic “Ionizing radiation”Dimensions: 960 x 720 pixels, format: jpg. To download a free slide for use in a physics lesson, right-click on the image and click “Save Image As...”. You can download the entire presentation “X-ray physics.ppt” in a 576 KB zip archive.
Download presentationIonizing radiation
“X-Ray Physicist” - January, 1896... But how? Head: Baeva Valentina Mikhailovna. Thus began the birth of the discovery. X-rays have the same properties as light rays. Discovery of X-rays. X-rays. The glow of the screen also disappeared; turned on the handset again - and the glow appeared again. In 1862, Wilhelm entered the Utrecht Technical School.
"Ultraviolet radiation" - Ultraviolet radiation. Radiation receivers. Biological action. High temperature plasma. Properties. The sun, stars, nebulae and other space objects. Ultraviolet radiation is divided into: For wavelengths less than 105 nm, there are practically no transparent materials. History of discovery. Photoelectric receivers are used.
"Infrared radiation" - Application. The warmer an object is, the faster it emits. Large doses may cause eye damage and skin burns. You can take photographs in ultraviolet rays (see Fig. 1). The earth emits infrared (thermal) radiation into the surrounding space. 50% of the solar radiation energy comes from infrared rays.
“Types of radiation physics” - During beta decay, an electron flies out of the nucleus. Chernobyl accident. The time it takes for half of the atoms to decay is called the half-life. Modern views on radioactivity. There are many different explanations for the causes of the Chernobyl accident. It turned out that the radiation is not uniform, but is a mixture of “rays”.