Oxidizer gives or accepts. The concept of the oxidizing agent and reducing agent
Fundamentals of theoretical chemistry
10. Redox reactions
Redox reactions in solutions.
Chemical reactions occurring with a change in the degree of oxidation of elements included in the reactant substances are called redox.
Oxidation
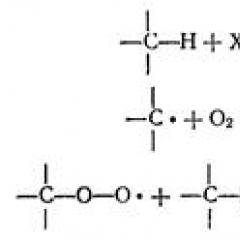
- this is the process of electron recoil atom, molecule or ion. If the atom gives its electrons, then it acquires a positive charge: l - , gives 1 electron, then it becomes a neutral atom:![]()
If a positively charged ion or an atom gives electrons, the value of its positive charge increases accordingly by the number of removable electrons:

Recovery is the process of connecting electrons by an atom, molecule or ion.
If the atom attachs electrons, it turns into a negatively charged ion:

If a positively charged ion receives electrons, then the value of its charge decreases:

or it can go to a neutral atom:

Oxidizer
receiving electrons. Restorener is an atom, molecule or ion, egging electrons.Oxidizing agent
in the process of reaction restores, the reducing agent is oxidized.It should be remembered that the consideration of oxidation (recovery) as the process of recoil (and the adoption) of electrons by atoms or ions does not always reflect the true position, since in many cases there is no complete transfer of electrons, but only the displacement of the electron cloud from one atom to another.
However, to compile equations of redox reactions, there is no significant value, which is formed - ion or covalent. Therefore, for simplicity, we will talk about attaching or disposable electrons regardless of the type of communication.
Determination of stoichiometric coefficients in the equations of redox reactions. In the preparation of the equation of the redox reaction, it is necessary to determine the reducing agent, oxidizing agent, and the number of devices and received electrons. As a rule, the coefficients are selected using either method electronic balance
, either the method electron-ion balance (sometimes the latter is called the method sereaction ).As an example of the compilation of equations of redox reactions, we consider the process of oxidation of pyrite with concentrated nitric acid.
First of all, we define the reaction products.
HNO 3. is a strong oxidant, so sulfur will be oxidized to the maximum degree of oxidation S 6+, and iron - to Fe 3+, with HNO 3 May restore beforeN0 and NO 2. We will choose N O:Where will be located
H 2 O. (in the left or right), we do not yet know.1. Apply first electron-ion balance method
(semoretosis). In this method, the transition of electrons from one atoms or ions to another, taking into account the nature of the medium (acid, alkaline or neutral), in which the reaction proceeds.In compiling the equations of oxidation and reduction processes for the equalization of the number of hydrogen atoms and oxygen, (depending on the medium) or water molecules and hydrogen ions are injected (depending on the medium) (if the environment is acidic), or water molecules and hydroxide ions (if alkaline environment). Accordingly, in the obtained products, hydrogen ions and water molecules (acid medium) or hydroxide ions and water molecules (alkaline medium) will be located in the right side of the electron-ion equation.
That is when writing electron-ion equations, it is necessary to proceed from the composition of the ions that are in solution.In addition, as in the preparation of abbreviated ion equations, the substances are slightly subsorative, poorly soluble or gas released you should write in molecular form.
Consider for our case half-formation of oxidation. Molecule
FES 2. turns into ion Fe 3+ (F E (N O 3) 3 completely dissociates on ions, hydrolysis neglect) and two ions SO 4 2. - (dissociation H 2 SO 4):In order to equalize oxygen in left part Add 8 molecules
2 Oh, and in the right - 16 ions + (Acute environment):The charge of the left side is 0, the charge is right +15, so
FES 2. Must give 15 electrons:Consider now half-rebellion of the recovery of nitrate ion:
![]()
It is necessary to take away
N O 3. 2 atoms O. To do this, add 4 ions to the left side 1+ (acid medium), and to right - 2 molecules 2 A:To equalize the charge to the left side (charge
+3) Add 3 electrons:Finally we have:

Reducing both parts on 16n
+ and 8n 2 O, we obtain a reduced ionic equation of the redox reaction:Adding the corresponding number of ions in both parts of the equation
NO 3. - and N +. We find the molecular reaction equation:Please note that to determine the number of given and received electrons, you never had to determine the degree of oxidation of the elements. In addition, we took into account the impact of the environment and automatically determined that
2 O is in the right part of the equation. Undoubtedly that this method much more corresponds to a chemical meaning than the standard electronic balance method, Although the last is somewhat easier for understanding.2. equalize this reaction by the method electronic balance . The recovery process is described:
![]()
It is more difficult to make a scheme of oxidation, since two elements are oxidized at once -
Fe and S. It is possible to attribute the degree of oxidation 2+, sulfur 1- and consider that one FE atom accounts for two atoms S:
You can, however, do without determining the degrees of oxidation and write a scheme that resembles a scheme
![]()
The right side has a charge of +15, left - 0, so
FES 2. must give 15 electrons. We write a common balance:
five NNO molecules
3 Go to oxidation FES 2, And three more molecules HNO 3. Needed for educationFe (N about 3) 3:To equalize hydrogen and oxygen, add to the right side two molecules
2 A:The method of electron-ion balance is more universal compared to the electronic balance method and has an indisputable advantage in selecting coefficients
in many redox reactions, in particular, involving organic compoundsin which even the procedure for determining the degrees of oxidation is very complex.Consider, for example, the process of ethylene oxidation occurring when passing it through water solution Permanganate potassium. As a result, ethylene is oxidized to ethylene glycol
CH 2 - CH 2 -One, and permanganate is restored to manganese oxide (IV), in addition, as will be apparent from the final balance equation, potassium hydroxide is also formed to the right:
After carrying out the necessary cuts of such members, write the equation in the final molecular form
Standard potentials of redox reactions.
The possibility of the flow of any oxidative-reducing reaction in real conditions is due to a number of causes: the temperature, the nature of the oxidizing agent and the reducing agent, the acidity of the medium, the concentration of substances involved in the reaction, etc. It is difficult to consider all these factors, but, remembering that Any oxidative-reducing reaction proceeds with the transfer of electrons from the reducing agent to the oxidizing agent, you can set the criterion for the possibility of flowing such a reaction.Quantitative characteristic of redox processes are normal oxidation and reduction potentials of oxidizing agents and reducing agents (or standard potentials electrodes).
In order to understand the physico-chemical meaning of such potentials, it is necessary to analyze the so-called electrochemical processes.
Chemical processes accompanied by occurrence electric current or caused by him, called electrochemical ones.
To understand the nature of electric chemical processesWe turn to the consideration of several sufficiently simple situations. Imagine a metal plate immersed in water. Under the action of polar water molecules, metal ions are removed from the surface of the plate and hydrated transitions to the liquid phase. The latter is charged positively, and an excess of electrons appears on the metal plate. The further process proceeds, the more the charge becomes
, both plates and liquid phases.Due to the electrostatic attraction of the cations of the solution and excess metal electrons on the border of the phase section, the so-called double electrical layer occurs, which slows down the further transition of metal ions into the liquid phase. Finally, the moment occurs when equilibrium is established between the solution and metal plate, which can be expressed by the equation:
or taking into account the hydration of ions in the solution:
The condition of this equilibrium depends on the nature of the metal, the concentration of its ions in the solution, on temperature and
pressure.When the metal is immersed, the equilibrium in accordance with the salt of this metal is shifted to the left and the higher the concentration of metal ions in the solution. Active metals whose ions have a good ability to switch to solution, will be charged negative in this case, although to a lesser extent than in clean water.
Equilibrium can be shown to the right, if one or another method of removing electrons from metal. This will lead to a dissolution of the metal plate. On the contrary, if it is from the outside to a metal plate, then it will precipitate ions
of solid.When the metal is immersed in the solution at the border of the phase section, a double electric layer is formed. The potential difference arising between the metal and the liquid phase surrounding it is called electrode potential. This potential is the characteristic of the oxidation and reduction capacity of the metal as a solid phase.
In an isolated metallic atom (the state of a single andatoma vapor, which occurs at high temperatures and high degrees of the vacuum), the reduction properties are characterized by another value called by ionization potential. The ionization potential is the energy required for the separation of an electron from an insulated atom.
The absolute value of the electrode potential cannot be measured directly. However, it is not difficult to measure the difference in electrode potentials, which occurs in a system consisting of two pairs metal - solution. Such couples are called semi-elements . It was agreed to determine the electrode potentials of metals with respect to the so-called standard hydrogen electrode, the potential of which is randomly accepted for zero. The standard hydrogen electrode consists of a specially prepared platinum plate, immersed in an acid solution with a concentration of hydrogen ions 1 mol / l and washed jet of gaseous hydrogen under pressure 10
5 PA, at a temperature of 25 ° C.A number of standard electrode potentials.
If the metal plate is immersed in a solution of its salt with a concentration of metal ions equal to 1 mol / l, combine with a standard hydrogen electrode, then a galvanic element will be obtained. The electromotive force of this element (EMF), measured at 25 ° C, and characterizes standard Electrode Metal Potential,denotable usually as E °.The standard potentials of electrodes acting as reducing agents with respect to hydrogen have a sign "-", and the "+" sign has the standard potentials of electrodes that are oxidizers.
Metals located in ascending order of their standard electrode potentials form the so-called electrochemical Metal Voltage Series : Li, RB, To, va, SR, Ca, Na, Mg, Al, Mn, Zn, Cr, Fe, CD, CO, NI, SN, PB, H, SB, BI, CU, HG, AG, PD, PT, AU.
A number of stresses characterize chemical properties Metals:
1. The larger the electrode potential of the metal is negative, the greater its restorative ability.
2. Each metal is able to exhibit (restore) from solutions of salts those metals that stand in an electrochemical row of metal voltages after it.
3. All metals having a negative standard electrode potential, i.e., located in the electrochemical row of metal voltages to the left of hydrogen, are able to exhibit it from acid solutions.
As in the case of determining the value of E ° of metals, the E ° of non-metals are measured at a temperature of 25 ° C and at the concentration of all atomic and molecular particles involved in equilibrium equal to 1 mol / l.
The algebraic value of the standard redox potential characterizes the oxidative activity of the corresponding oxidized form. therefore comparison of the values \u200b\u200bof standard redox potentials allows you to answer the question: whether such or another redox reaction takes place?
Quantitative criterion for assessing the possibility of flowing one or another redox reaction is the positive value of the difference in standard oxidation and recovery potentials of oxidation and recovery semi-resources.
Electrolysis solutions.
The combination of redox reactions that flow on electrodes in solutions or electrolyte melts when the electric current is passed through them, is called electrolysis.At the cathode of the current source, the process of transmitting electrons by cations from a solution or melt, so the cathode is a "reducing agent". On the anode there is a return of electrons by anions, so anode is a "oxidizing agent".
When electrolysis, both competing processes may occur on the anode and on the cathode.
When conducting electrolysis using an inert (unsuitable) anode (for example, graphite or platinum), as a rule, two oxidative and two rehabilitation process are competing:
on the anode - oxidation of anions and hydroxide ions,
at the cathode - restoration of cations and hydrogen ions.
When carrying out electrolysis using the active (consumed) anode, the process is complicated and competing reactions on the electrodes are:
on the anode - the oxidation of anions and hydroxide ions, the anode dissolution of the metal - the anode material;
at the cathode - restoration of the cation of salt and hydrogen ions, the restoration of metal cations obtained by dissolving the anode.
When choosing the most likely process on the anode and cathode, it should be proceeded from the position that the reaction will occur for which the lowest energy costs is required. In addition, to select the most likely process on an anode and cathode with electrolysis of solutions of salts with an inert electrode, the following rules use:
The anode can form the following products: a) with electrolysis of solutions containing in its composition anions f -, SO 4 2-, NAbout 3. - , PO 4 3. - , as well as alkalis solutions, oxygen is released; b) when anon oxidation withl. - , In R. -, I- The chlorine, bromine, iodine, respectively;c) When oxidizing the anions of organic acids, the process takes place:2. With electrolysis of solutions of salts containing ions located in a row of voltages to the left AL
3+ , hydrogen is distinguished on the cathode; If the ion is located in a row of stresses to the right of hydrogen, then the metal is allocated on the cathode.3. With electrolysis of solutions of salts containing ions located in a row of stresses between
Al + and H + At the cathode, competing processes of both restoration of cations and hydrogen is released.Consider as an example electrolysis of the aqueous solution of copper chloride on the inert electrodes. In solution are si ions
2+ and 2SL -, which under the action of electric current are sent to the corresponding electrodes:
Metal copper is released on the cathode, chlorine gaseous gas on the anode.
If in the considered example of the electrolysis of the solution
CUCL 2. As anode, take a copper plate, then copper stands out on the cathode, and on the anode where oxidation processes occur, instead of the discharge of ions withl. - And chlorine isolation occurs oxidation of the anode (copper). In this case, the dissolution of the anode itself, and in the form of si ionsgoes into solution. Electrolysis CUCL 2. With soluble anode, you can record this:
Electrolysis solutions of salts with soluble anode comes down to the oxidation of the material of the anode (its dissolution) and is accompanied by the transfer of metal from the anode to the cathode. This property is widely used in refining (cleaning) of metals from contamination.
Electrolysis of melts. To obtain highly active metals (sodium, aluminum, magnesium, calcium, etc.), the electrolysis of melt salts or oxides is used to be interacting with water;

If you pass the electric current through an aqueous solution of active metal salts and oxygen-containing acid, neither metal cations nor the ions of the acid residue are not discharged. Hydrogen is distinguished on the cathode,
a. anode is oxygen, and electrolysis comes down to electrolytic water decomposition.Electrolysis of electrolyte solutions to carry out is energy more energy than melt, since electrolytes - salts and alkali - melt at very high temperatures.
Faraday electrolysis law.
The dependence of the amount of substance formed under the action of electric current, the current, current and the nature of the electrolyte can be installed on the basis of the generalized faraday law :where t - Mass formed under electrolysis substances (g); E - equivalent mass of substance (g / mol); M - molar mass substances (g / mol); p - the number of devices given or received electrons;
I - current (a); t - Duration of the process(from); F - Faraday Constant,characterizing the amount of electricity required for the release of 1 equivalent mass of the substance(F \u003d. 96 500 CL / mol \u003d 26.8 A × h / mol).These include reactions in which the reacting substances are exchanged by electrons by changing the degree of oxidation of the atoms of the elements that are part of the reacting substances.
For example:
Zn + 2h + → zn 2+ + h 2,
FES 2 + 8HNO 3 (conc.) \u003d Fe (NO 3) 3 + 5NO + 2H 2 SO 4 + 2H 2 O,
The overwhelming majority of chemical reactions refer to redox, they play an extremely important role.
Oxidation is the process of recoil electron atom, molecule or ion.
If the atom gives its electrons, then it acquires a positive charge:
For example:
Al - 3e - \u003d Al 3+
H 2 - 2E - \u003d 2H +
When oxidation, the degree of oxidation increases.
If a negatively charged ion (charge -1), for example, Cl -, gives 1 electron, then it becomes a neutral atom:
2CL - - 2E - \u003d CL 2
If a positively charged ion or an atom gives electrons, the value of its positive charge increases accordingly by the number of removable electrons:
Fe 2+ - E - \u003d Fe 3+
Recovery is the process of connecting electrons by an atom, molecule or ion.
If the atom attaches electrons, it turns into a negatively charged ion:
For example:
CL 2 + 2E- \u003d 2SL -
S + 2e - \u003d S 2-
If a positively charged ion receives electrons, then the value of its charge decreases:
Fe 3+ + E- \u003d Fe 2+
or it can go to a neutral atom:
Fe 2+ + 2e- \u003d Fe 0
The oxidizing agent is an atom, molecule or ion accepting electrons. The reducing agent is an atom, molecule or ion, producing electrons.
The oxidizing agent is restored during the reaction, the reducing agent is oxidized.
Oxidation is always accompanied by restoration, and vice versa, recovery is always associated with oxidation, which can be expressed by the equations:
Restorener - E - ↔ Oxidizer
Oxidizer + E - ↔ Restore
Therefore, oxidative reaction reactions are the unity of two opposite processes - oxidation and recovery.
Major reducing agents and oxidizers
|
Restores |
Oxidifiers |
Metals, hydrogen, coal Carbon (II) CO oxide Hydrogen sulfide H 2 S, sulfur oxide (IV) SO 2, sulfuric acid H 2 SO 3 and its salts Hi hydrogen-hydrogen acid, HBr bromomic acid, hydrochloric acid HCL SNCL 2 chloride, iron (II) sulfate FESO 4, manganese sulfate (II) MNSO 4, chromium sulfate (III) CR 2 (SO 4) 3 Azobic acid HNO 2, ammonia NH 3, hydrazine N 2 H 4, nitrogen oxide (II) NO Phosphoric acid H 3 PO 3 Aldehydes, alcohols, ant and oxalic acids, glucose Cathode at electrolysis |
Halogens Potassium permanganate KMNO 4, potassium manganate K 2 MNO 4, manganese oxide (IV) MNO 2 Dichromat potassium K 2 Cr 2 O 7, potassium chromat K 2 CRO 4 Nitric Acid HNO 3 Oxygen O 2, ozone about 3, hydrogen peroxide H 2 O 2 Sulfuric acid H 2 SO 4 (conc.), Seleous acid H 2 SEO 4 Copper Oxide (II) Cuo, Silver Oxide (I) AG 2 O, Lead Oxide (IV) PBO 2 Ions of noble metals (AG +, AU 3+, etc.) Iron chloride (III) FECL 3 Hypochlorites, chlorates and perchlorates Tsarist Vodka, a mixture of concentrated nitric and plating acids Anode with electrolysis |
Electronic balance method.
For the equalization of the OSR, several methods are used, from which we while consider one - the electronic balance method.
Write the reaction equation between aluminum and oxygen:
Al + O 2 \u003d Al 2 O 3
Let it be misleading the simplicity of this equation. Our task is to deal with the method that in the future will allow you to equalize much more complex reactions.
So, what is the method of electronic balance? Balance is equality. Therefore, it should be made the same amount of electrons that one element gives and receives another element in this reaction. Initially, this number looks different, which can be seen from different degrees Aluminum and oxygen oxidation:
Al 0 + O 2 0 \u003d Al 2 +3 O 3 -2
Aluminum gives electrons (acquires a positive degree of oxidation), and oxygen - accepts electrons (acquires a negative degree of oxidation). To obtain the degree of oxidation +3, the aluminum atom must pay 3 electrons. Oxygen molecule to turn into oxygen atoms with a degree of oxidation -2, should take 4 electrons:
Al 0 - 3e- \u003d Al +3
O 2 0 + 4E- \u003d 2O -2
In order for the number of given and received electrons to be leveled, the first equation must be multiplied by 4, and the second - by 3. To do this, it is enough to move the numbers of the given and received electrons against the upper and lower lines as shown in the circuit at the top.
If now in the equation before the reducing agent (AL) we will put the coefficient 4 we found, and in front of the oxidizing agent (O 2) - the coefficient we found by us 3, the number of given and received electrons is aligned and becomes equal to 12. The electronic balance is reached. It can be seen that in front of the reaction product Al 2 O 3 requires a coefficient 2. Now the equation of the redox reaction is equal:
4Al + 3O 2 \u003d 2AL 2 O 3
All advantages of the electronic balance method are manifested in more complex cases than oxygen oxygen oxygen.
For example, known to all "manganese" - manganese-oxidant potassium KMNO 4 is a strong oxidizing agent due to the Mn atom to the degree of oxidation +7. Even Anion Chlorine CL - gives it an electron, turning into a chlorine atom. This is sometimes used to obtain chlorine gaseous chlorine in the laboratory:
K + Mn +7 O 4 -2 + K + Cl - + H 2 SO 4 \u003d Cl 2 0 + Mn +2 SO 4 + K 2 SO 4 + H 2 O
We will make an electronic balance sheet scheme:
Mn +7 + 5e- \u003d Mn +2
2CL - - 2E- \u003d Cl 2 0
Two and five are the main coefficients of the equation, thanks to which it is easy to choose all other coefficients. Before CL 2, put the coefficient 5 (or 2 × 5 \u003d 10 before the KCL), and before KMNO 4 - coefficient 2. All other coefficients are tied to these two coefficients. It is much easier than to act a simple number of numbers.
2 KMNO 4 + 10KCl + 8H 2 SO 4 \u003d 5 Cl 2 + 2mnso 4 + 6k 2 SO 4 + 8H 2 O
To equalize the number of atoms to (12 atoms on the left), it is necessary before K 2 SO 4 in the right part of the equation to put the coefficient 6. Finally, to equate oxygen and hydrogen, sufficiently in front of H 2 SO 4 and H 2 O put the coefficient 8. We received an equation In final form.
The electronic balance method, as we see, does not exclude the ordinary selection of coefficients in the equations of redox reactions, but it may noticeably alleviate such a selection.
Compilation of the equation of reaction of copper with a solution of palladium nitrate (II). We write the formula of the initial and finite substances of the reaction and show the changes in the degrees of oxidation:
it follows that when the reducing and oxidizer, the coefficients are equal to 1. The final response equation:
Cu + Pd (NO 3) 2 \u003d Cu (NO 3) 2 + PD
As can be seen, in the total reaction equation, the electrons do not appear.
To check the correctness of the composed equation, we count the number of atoms of each element in its right and left parts. For example, in the right part of 6 oxygen atoms, in the left also 6 atoms; palladium 1 and 1; Copper is also 1 and 1. So the equation is compiled correctly.
Rewrite this equation in ion form:
Cu + PD 2+ + 2NO 3 - \u003d Cu 2+ + 2NO 3 - + PD
And after cutting the same ions we get
Cu + PD 2+ \u003d Cu 2+ + Rd
Drawing up the equation of the reaction of the interaction of manganese oxide (IV) with concentrated hydrochloric acid
(With the help of this reaction in the laboratory, chlorine is obtained).
We write the formula of the initial and finite substances of the reaction:
NCl + MNO 2 → SL 2 + MNSL 2 + H 2 O
We show the change in the degrees of the oxidation of atoms before and after the reaction:
This reaction is oxidative and reductive, as the degree of oxidation of chlorine and manganese atoms change. NCL - reducing agent, MNO 2 - oxidizing agent. We compile electronic equations:

and we find the coefficients during the reducing and oxidizer. They are respectively 2 and 1. The coefficient 2 (and not 1) is concluded because 2 chlorine atoms with a degree of oxidation -1 give 2 electrons. This coefficient is already in the electronic equation:
2NSL + MNO 2 → SL 2 + MNSL 2 + H 2 O
We find coefficients for other reacting substances. From electronic equations it can be seen that at 2 mole HCl accounts for 1 mol MNO 2. However, given that, for the binding of the generated two-chart, the manganese need 2 more mol of acids, before the reducing agent, the coefficient should be put 4. Then the water will be 2 mol. The final equation has the form
4NCl + MNO 2 \u003d SL 2 + MNSL 2 + 2N 2
Checking the correctness of the writing equation can be limited by counting the number of atoms of a single element, for example chlorine: in the left side 4 and in the right 2 + 2 \u003d 4.
Since the electronic balance method shows the equations of reactions in the molecular form, then after compiling and checking, they should be written in ion form.
We rewrite the composed equation in ion form:
4H + + 4Cl - + mno 2 \u003d CL 2 + Mn 2 + 2SL - + 2N 2 o
and after reducing the same ions in both parts of the equation
4N + + 2SL - + MNO 2 \u003d CL 2 + Mn 2 + + 2n 2
Compilation of the equation of the reaction of the interaction of hydrogen sulfide with an acidified solution of potassium permanganate.
We write the reaction scheme - formulas of the initial and obtained substances:
H 2 S + kmno 4 + H 2 SO 4 → S + MNSO 4 + K 2 SO 4 + H 2 O
Then show the change in the degrees of the oxidation of atoms before and after the reaction:
The degrees of oxidation of sulfur atoms and manganese (H 2 S - the reducing agent, KMNO 4 - oxidizing agent). We compile electronic equations, i.e. We depict the processes of return and addition of electrons:
Finally, we find coefficients for oxidizing and reducing, and then with other reacting substances. From the electronic equations it can be seen that it is necessary to take 5 mol H 2 S and 2 mole KMNO 4, then we obtain 5 mol of atoms S and 2 mens MNSO 4. In addition, from comparison of atoms in the left and right parts of the equation, we will also find that 1 mol to 2 SO 4 and 8 mole of water is also formed. The final response equation will be viewed
5n 2 s + 2kmno 4 + zn 2 SO 4 \u003d 5S + 2MNSO 4 + K 2 SO 4 + 8N 2
The correctness of the writing of the equation is confirmed by the counting of atoms of one element, for example oxygen; In the left side of their 2 4 + 3 4 \u003d 20 and in the right portion 2 4 + 4 + 8 \u003d 20.
Rewrite the equation in ion form:
5n 2 s + 2mno 4 - + 6h + \u003d 5s + 2mn 2+ + 8N 2 o
It is known that the correctly written reaction equation is an expression of the law of preserving the mass of substances. Therefore, the number of one and the same atoms in the initial substances and reaction products should be the same. Charges should be maintained. The amount of charge charges should always be equal to the amount of charge charges of the reaction.
The electron-ion balance method is more universal compared to the electronic balance method and has an indisputable advantage in the selection of coefficients in many oxidation reactions, in particular, with the participation of organic compounds in which even the procedure for determining oxidation degrees is very complex.
Classification of Ovr.
There are three basic types of redox reactions distinguish:
1) Reactions of intermolecular oxidation-recovery
(when the oxidizer and reducing agent are different substances);
2) Disproportionation reactions
(when the same substance can be the oxidizing and reducing agent);
3) reactions of intramolecular oxidation-recovery
(When one part of the molecule acts as an oxidizing agent, and the other - as a reducing agent).\u003e
Consider examples of the reactions of three types.
1. Reactions of intermolecular oxidation-restoration are all reactions already considered by us in this paragraph.
Consider a few more difficult caseWhen not the whole oxidizer can be consumed in the reaction, since part of it is involved in the usual - non-oxidative and reductional exchange reaction:
Cu 0 + H + N +5 O 3 -2 \u003d Cu +2 (n +5 O 3 -2) 2 + N +2 O -2 + H 2 O
Part of the NO 3 particles is involved in the reaction as an oxidizing agent, giving nitrogen oxide NO, and a portion of NO 3 ions is unchanged in a compound of copper Cu (NO 3) 2. Make an electronic balance:
Cu 0 - 2e- \u003d Cu +2
N +5 + 3e- \u003d n +2
We deliver the coefficient 3 facing Cu and Cu (NO 3) 2. But the coefficient 2 should be put only before NO, because all the nitrogen available in it participated in the oxidative reaction reaction. It would be an error to put the coefficient 2 before HNO 3, because this substance includes both nitrogen atoms that do not participate in the reduction oxidation and are part of the Cu product (NO 3) 2 (NO 3 particles - sometimes called "ion -Harmiter ").
The remaining coefficients are closed without difficulty:
3 CU + 8HNO 3 \u003d 3 CU (NO 3) 2 + 2 NO + 4H 2 O
2. The disproportionation reactions occur when the molecules of the same substance are able to oxidize and restore each other. This becomes possible if the substance contains the atoms of any element in the intermediate degree of oxidation.
Consequently, the degree of oxidation is capable of both falling, and increase. For example:
HN +3 O 2 \u003d HN +5 O 3 + N +2 O + H 2 O
This reaction can be represented as a reaction between HNO 2 and HNO 2 as an oxidizing agent and a reducing agent and apply an electronic balance method:
HN +3 O 2 + HN +3 O 2 \u003d HN +5 O3 + N +2 O + H 2 O
N +3 - 2e- \u003d n +5
N +3 + e- \u003d n +2
We get the equation:
2HNO 2 + 1HNO 2 \u003d 1 HNO 3 + 2 NO + H 2 O
Or, folding together moli HNO 2:
3HNO 2 \u003d HNO 3 + 2NO + H 2 O
The reactions of intramolecular oxidation-reduction occur when the oxidant atoms and reducing atoms are adjacent in the molecule. Consider the decomposition of the KCLO 3 beverage salt when heated:
KCL +5 O 3 -2 \u003d KCL - + O 2 0
This equation is also subject to the requirement of the electronic balance:
Cl +5 + 6e- \u003d Cl -
2O -2 - 2E- \u003d O 2 0
Here there is a difficulty - which of the two factors found in front of KCLO 3 - after all, this molecule contains an oxidizing agent and reducing agent?
In such cases, the factors found are placed in front of the products:
KCLO 3 \u003d 2KCL + 3O 2
Now it is clear that before KCLO 3 you need to put the coefficient 2.
2KCLO 3 \u003d 2KCL + 3O 2
The intramolecular reaction of the decomposition of the bertolen salt during heating is used when the oxygen is obtained in the laboratory.
Semi-resource method

As the name itself shows, this method is based on the preparation of ion equations for the oxidation process and the recovery process, followed by summing them in the general equation.
As an example, the equation of the same reaction was used with the explanation of the electronic balance method.
When sinking sulfide H 2 S through the acidified solution of potassium permanganate KMNO 4, the raspberry color disappears and the solution is muttered.
Experience shows that the cloudiness of the solution occurs as a result of the formation of elemental sulfur, i.e. Process flow:
H 2 S → S + 2H +
This scheme is equalized by the number of atoms. To equalize the number of charges, it is necessary to take two electron from the left side of the circuit, after which it is possible to replace the arrow to sign equal:
H 2 S - 2e - \u003d S + 2H +
This is the first half-formation - the process of reducing agent H 2 S.
The decolorization of the solution is associated with the transition of the MNO 4 ion - (it has a raspberry color) to the Mn 2+ ion (almost colorless and only with a large concentration has a weakly pink color) that can be expressed by the scheme
MNO 4 - → Mn 2+
In an acidic solution, oxygen, which is part of MNo ions, together with hydrogen ions ultimately forms water. Therefore, the transition process is written as follows:
MNO 4 - + 8H + → Mn 2+ + 4N 2 o
In order for the arrow to replace the equal sign, you need to equalize and charges. Since the initial substances have seven positive charges (7+), and the final - two positive (2+), then to perform the conditions for the preservation of charges, it is necessary to add five electrons to the left side of the circuit:
MNO 4 - + 8H + + 5e - \u003d Mn 2+ + 4N 2
This is the second half-reaction - the process of restoring the oxidant, i.e. Permanganate-ion.
For compilation general equation The reaction should be the half-reaction equations of reassessing reacts, pre-equalizing the number of removable and obtained electrons. In this case, according to the rules of finding the smallest multiple, the corresponding multipliers are determined to which the half-reaction equations are multiplied. Abbreviated recording is carried out like this:

And, shining for 10n +, finally get
5n 2 s + 2mno 4 - + 6h + \u003d 5s + 2mn 2+ + 8N 2 o
We check the correctness of the equation composed in the ion form: the number of oxygen atoms in the left side 8, in the right 8; The number of charges: in the left part (2 -) + (6+) \u003d 4+, in the right 2 (2+) \u003d 4+. The equation is properly compiled, since atoms and charges are equalized.
The half-formation method contains the reaction equation in ion form. To move from it to the molecular form equation, we do this: on the left side of the ionic equation, the corresponding cation is selected for each anion, and an anion anion to each cation. Then the same ions in the same number are recorded in the right-hand part of the equation, after which the ions are combined into the molecules:

Thus, the compilation of equations of oxidation reactions using the half-reaction method leads to the result that the electronic balance method.
Comparison both methods. The advantage of the semi-reaction oteoda compared to the electronic balance method is. that it does not use hypothetical ions, but really existing ones. In fact, there are no ions in the solution, and there are ions.
With the method of half-formations, you do not need to know the degree of oxidation of atoms.
The writing of certain ionic equations of semoretosis is necessary to understand the chemical processes in the electroplating element and at electrolysis. At the same time, the method is visible to the role of the environment as an active participant in the entire process. Finally, when using the half-formation method, you do not need to know all the resulting substances, they appear in the reaction equation when it is derived. Therefore, the half-formation method should be preferred and used in the preparation of equations of all redox reactions occurring in aqueous solutions.
Many substances have special properties that are called oxidative or recoverable in chemistry.
Some chemicals exhibit the properties of oxidants, other - reducing agents, while some compounds can show those and other properties at the same time (for example - hydrogen peroxide H 2 O 2).
What is the oxidizing agent and reducing agent, oxidation and recovery?
The redox properties of the substance are associated with the process of returning and taking electrons by atoms, ions or molecules.
The oxidizer is a substance that electrons receives during the reaction, i.e. is restored; The reducing agent - gives electrons, i.e. it is oxidized. The transmission processes of electrons from one substances to others are usually called redox reactions.
Compounds containing atoms of elements with the maximum degree of oxidation can only be oxidizing at these atoms, because They already gave all their valence electrons and are able to only take electrons. The maximum degree of oxidation of an element atom is equal to the number of the group in the periodic table to which this item belongs. Compounds containing the atoms of elements with a minimum degree of oxidation can only serve as reducing agents, since they can only give electrons, because the external energy level in such atoms is completed by eight electrons
With chemical reactions, the number and nature of the links between interacting atoms may vary, i.e. The degrees of oxidation of atoms in molecules may vary.
Reactions, resulting in which the degrees of oxidation of atoms are changed, are called redox.
Examples of redox reactions (abbreviated OSR):
Changes in the degree of oxidation is associated with displacement or electron transmission. Regardless of whether electrons are moving from one atom to another or only partially delayed by one of the atoms, it is conventionally indicated about the return and addition of electrons.
Processreturn electrons at an atom or ion calledoxidation . Processattachment electrons are calledrestoration .
Substances, atoms or ions of which give electrons, are called restores . During the reaction, they are oxidized. Substances, atoms or ions of which are attached electrons are called oxidifiers . During the reaction, they are restored.
The oxidation and recovery processes are depicted by electronic equations, which indicates the change in the degree of oxidation of interacting atoms and the number of electrons given by the reducing agent or accepted by the oxidizing agent.
Examples of equations expressing oxidation processes:

Equations expressing recovery processes:

Redox reaction is a single process in which oxidation and recovery proceed at the same time. The oxidation of one atom is always accompanied by the restoration of the other and vice versa. Wherein general the number of electrons given by the reducing agent is equal to the number of electrons connected by the oxidizing agent.
In accordance with the law of equivalents the masses of reacting substances belong to each other as molar masses of their equivalents. The equivalent amount of substance in the OSR depends on the number of electrons given or atoms connected by it; The molar mass of the equivalent is calculated by the formula:
 , (1)
, (1)
where M. - molar mass of substance, g / mol
M. ek. - molar mass of equivalent of substances, g / mol
 -Enecluded or attached. Elelectrons
-Enecluded or attached. Elelectrons
For example, in the reaction
atom manganese attachs 5 electrons, so equivalent  is 1/5. molea sulfur atom gives 2 electrons and equivalent
is 1/5. molea sulfur atom gives 2 electrons and equivalent  is 1/2. mole. The molar masses of equivalents are respectively equal
is 1/2. mole. The molar masses of equivalents are respectively equal
Types of oxidation reaction
There are three types of chemical OSPs: intermolecular, intramolecular and self-examination reactions. A separate group consists of electrochemical reactions.
1. Intermolecular oh is reactions in which the oxidizing agent and the reducing agent are different substances:

2. Intramolecular oh is reactions in which the degrees of oxidation of different atoms of one molecule are changing:

3. The reactions of self-substitution-self-healing are reactions in which the oxidation and restoration of atoms of the same element occurs:

4. Electrochemical reactions are AURO, in which oxidation and reduction processes are separated spatially (proceeding on separate electrodes), and the electrons are transmitted from the reducing agent to the oxidizing agent for an external electrical circuit:
Redox reactions are usually complex, but, knowing the formulas of the reagents and reaction products and can determine the degrees of oxidation of atoms, one can easily separate the coefficients in the equation of any HSR.
Redox reactions, or abbreviated OSR, are one of the foundations of the subject of chemistry, as they describe the interaction of individual chemical elements together. As follows from the name of these reactions, at least two different are involved in them. chemicals One of which acts as an oxidizing agent, and the other is a reducing agent. Obviously, it is very important to be able to distinguish and determine them in various chemical reactions.
How to determine the oxidizer and reducing agentThe main difficulty in determining the oxidizing agent and the reducing agent in chemical reactions is that the same substances in different cases can be both oxidizing agents and reducing agents. To learn how to correctly determine the role of a particular chemical element in the reaction, the following basic concepts should be clearly understood.
- Oxidation Call the process of electron recoil from the outer electronic layer of the chemical element. In turn oxidizer there will be an atom, molecule or ion that receive electrons and thereby lower their oxidation, which is restore . After the chemical reaction of interaction with another substance, the oxidant always acquires a positive charge.
- Restoration Call the process of connecting electrons to an outer electronic layer of the chemical element. Restorener there will be an atom, molecule or ion that give their electrons and thereby increase the degree of their oxidation, that is oxidize . After the chemical reaction of interaction with another substance, the reducing agent always acquires a positive charge.
- Simply put the oxidizer is a substance that "selects" electrons, and the reducing agent is a substance that gives them to the oxidizing agent. To determine who in the oxidation reaction performs the role of an oxidizing agent, who is a reducing agent and in what cases the oxidizing agent becomes a reducing agent and, on the contrary, can be known for typical behavior in the chemical reactions of individual elements.
- Typical reducing agents are metals and hydrogen: Fe, K, Ca, Cu, Mg, Na, Zn, h). The smaller they are ionisiroans, the more their rehabilitation properties. For example, partially oxidized iron, which has given one electron and having +1, will be able to give one electron less compared to the "clean" hardware. Also reducing agents may be compounds of chemical elements in low degree Oxidations that are filled with all free orbital and which can only give electrons, such as Ammonia NH 3, hydrogen sulfide H 2 S, HRB bromomarodamine, iodine hydrogen Hi, HCL chloride.
- Typical oxidants are many non-metals (F, CL, I, O, BR). Also oxidants can perform metals having a high degree of oxidation (Fe +3, Sn +4, Mn +4), also some elements connections in high degree Oxidation: Potassium permanganate KMNO 4, sulfuric acid H 2 SO 4, nitric acid HNO 3, copper oxide Cuo, iron chloride FECL 3.
- Chemical compounds In incomplete or intermediate degrees of oxidation, for example, single-axis nitric acid HNO 2, hydrogen peroxide H 2 O 2, sulfuric acid H 2 SO 3 may exhibit both oxidative and rehabilitation properties depending on the redox properties of the participating second reagent.

Ka follows this example One sodium atom gives one atom of the oxygen its electron. Consequently, sodium is a reducing agent, and oxygen by oxidizer. In this case, the sodium will fall completely, as it will give the maximum possible amount of electrons, and the oxygen atom will not be restored, as one more electron from another oxygen atom can take.