Physical and chemical methods of obtaining nanosystems. Technology obtaining nanomaterials
To date, a large number of methods and methods for obtaining nanomaterials have been developed. This is due to a variety of composition and properties of nanomaterials, on the one hand, and on the other, it allows you to expand the range of this class of substances, create new and, unique samples. The formation of nanoscale structures can occur in the course of processes such as phase transformations, chemical interaction, recrystallization, amorphization, high mechanical loads, biological synthesis. As a rule, the formation of nanomaterials is possible in the presence of significant deviations from the equilibrium conditions of the existence of a substance, which requires the creation of special conditions and, often, complex and precision equipment. Improving the previously known and developing new methods for obtaining nanomaterials determined the basic requirements that they must comply with, namely:
the method must ensure the material of the controlled composition with the reproducible properties;
the method must ensure the temporary stability of nanomaterials, i.e. First of all, the protection of the surface of the particles from spontaneous oxidation and sintering during the manufacturing process;
the method must have high performance and efficiency;
the method should ensure nanomaterials with a certain particle size or grains, and their distribution in size must be, if necessary, quite narrow.
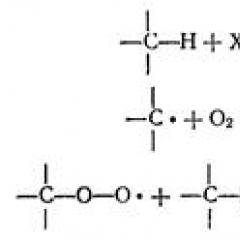
It should be noted that at present there is no method that meets the full set of requirements. Depending on the method of obtaining such characteristics of nanomaterials, such as the average size and shape of the particles, their particle size distribution, the value of the specific surface, the content of impurities in them, etc., can fluctuate in very wide limits. For example, nanopowders depending on the method and production conditions may have spherical, flake, needle or spongy shape; Amorphous or fine-crystal structure. Methods for obtaining nanomaterials are divided into mechanical, physical, chemical and biological. Those. This classification is based on the nature of the synthesis of nanomaterials. The basis of mechanical methods of obtaining is the impact of large deforming loads: friction, pressure, pressing, vibration, cavitation processes, etc. Physical methods of obtaining are based on physical transformations: evaporation, condensation, sublimation, sharp cooling or heating, spraying melt, etc. The chemical includes methods, the main dispersing step of which are: electrolysis, recovery, thermal decomposition. Biological methods of obtaining are based on the use of biochemical processes occurring in protein bodies. Methods of mechanical grinding in relation to nanomaterials are often called mechanoNTEZ. The basis of the mechanosintite is the mechanical processing of solids. Mechanical impact when grinding materials is impulse, i.e. The occurrence of the field of stress and its subsequent relaxation occurs not during the entire time of the residence of particles in the reactor, but only at the time of the collision of the particles and in a short time after it. The mechanical impact is also local, as it does not occur in the entire mass of the solid substance, and where the voltage field occurs and then relaxes. Due to the impulse and locality in small areas of the material for a short time, large loads are focused. This leads to the emergence of defects, stresses, shear strips, deformations, cracks. As a result, the grinding of the substance occurs, mass transfer is accelerated and mixing components, the chemical interaction of solid reagents is activated. As a result of mechanical abrasion and mechanical fusion, a higher mutual solubility of certain elements in solid state can be achieved than possible in equilibrium conditions. Grinding is carried out in ball, planetary, vibration, vortex, gyroscopic, inkjet mills, attributes. Grinding in these devices occurs as a result of shocks and abrasion. A variety of mechanical grinding method is a mechanochemical method. With a thin grinding of a mixture of various components between them, interaction is accelerated. In addition, there may be chemical reactions that, when contacting, not accompanied by grinding, do not occur at all at such temperatures. These reactions are called mechanochemical. In order to form a nanostructure in bulk materials, special mechanical deformation schemes are used, which make it possible to achieve large distortion of the structure of the samples at relatively low temperatures. Accordingly, the following methods include intensive plastic deformation: - high pressure twisting; - equational angular pressing (RCU-pressing); - method of comprehensive forging; - equational angular extract (RCU-extract); - method of "hourglass"; - Intensive friction method with sliding. Currently, most of the results are obtained by the first two methods. Recently, methods of obtaining nanomaterials using mechanical impact Different media. These methods include cavitation-hydrodynamic, vibration methods, a shock wave method, ultrasound grinding and detonation synthesis. The cavitation and hydrodynamic method is used to obtain nanopowders suspensions in various dispersion media. Cavitation - from Lat. The words "emptiness" - formation in liquid cavities (cavitation bubbles or cavities) filled with gas, ferry or mixture thereof. In the course of the process, cavitation effects caused by the formation and destruction of the vapor-gas microbubbles in the liquid for 10-3 - 10-5 s at pressures of the order of 100-1000 MPa, lead to warming not only liquids, but also solid tel . This impact causes the grinding of solid particles. The grinding of ultrasound is also based on the precipitation of cavitation strikes. The vibration method for obtaining nanomaterials is based on the resonant nature of effects and phenomena, which provide minimal energy consumption during processes and a high degree of homogenization of multiphase media. The principle of operation is that any vessel is subjected to a vibrational effect with a certain frequency and amplitude. Almaz nanoparticles can be obtained by detonation synthesis. The method uses the energy of the explosion, while the pressure is achieved in hundreds of thousands of atmospheres and temperatures up to several thousand degrees. These conditions correspond to the area of \u200b\u200bthermodynamic stability of the diamond phase. Physical methods of obtaining materials include spraying methods, condensation evaporation processes, vacuum sublimation technologies, techniques in solid state. The method of spraying the melt stream with liquid or gas is that the thin jet of the liquid material is supplied to the chamber, where it is broken into small drops with a stream of compressed inert gas or a fluid jet. As gases in this method use argon or nitrogen; As liquids - water, alcohols, acetone, acetaldehyde. The formation of nanostructures is possible by managing a liquid state or spinning. The method consists in obtaining thin ribbons using a quick (at least 106 k / s) melt cooling on the surface of the rotating disk or drum. Physical methods. Methods of evaporation-condensation are based on the preparation of powders as a result of the steam transition - a solid body or steam - a solid body in a gas volume or on a cooled surface. The essence of the method is that the starting material evaporates through intense heating, and then cooled sharply. The heating of the evaporated material can be carried out in various ways: resistive, laser, plasma, electric arc, induction, ionic. The process of evaporation condensation can be carried out in vacuo or neutral gas medium. The electric explosion explosion is carried out in argon or helium at a pressure of 0.1 - 60 MPa. In this method, thin metal wires with a diameter of 0.1 - 1 mm are placed in the chamber and the current of high force is impulse. The duration of the pulse is 10-5 - 10-7 s, the current density is 104-106 A / mm 2. At the same time, the wires are instantly warmed up and explode. The formation of particles occurs in a free flight. Vacuum sublimation technology of obtaining nanomaterials includes three main stages. In the first stage, the original solution of the treated substance or several substances is prepared. The second stage - the freezing of the solution - aims to fix the uniform spatial distribution of the components inherent in fluids for minimally possible size Crystallites in solid phase. The third stage is the removal of solvent crystallites from a frozen solution by sublimation. There are a number of methods for obtaining nanomaterials in which dispersion is carried out in a solid matter without changing aggregate state. One method of obtaining massive nanomaterials is a method of controlled crystallization from an amorphous state. The method involves obtaining an amorphous material by hardening from a liquid state, and then in conditions of controlled heating, a crystallization of the substance is carried out. Currently the most common method of obtaining carbon nanotubes is the method of thermal spraying of graphite electrodes in the plasma arc discharge. The synthesis process is carried out in a chamber filled with high pressure helium. When the plasma is burning, there is an intense thermal evaporation of the anode, while the precipitate is formed on the end surface of the cathode, in which carbon nanotubes are formed. The resulting numerous nanotubes have a length of about 40 microns. They grow on a cathode perpendicular to the flat surface of its end and are collected in cylindrical beams with a diameter of about 50 μm. Nanotube beams regularly cover the surface of the cathode, forming a cellular structure. It can be detected by considering the precipitate on the cathode with the naked eye. The space between the nanotube beams is filled with a mixture of disordered nanoparticles and single nanotubes. The content of nanotubes in the carbon sediment (deposit) can approach 60%. Chemical methods for obtaining nanoscale materials can be divided into groups in one of which can include methods where the nanomaterial is obtained by a particular chemical reaction in which certain classes of substances are involved. Another options for electrochemical reactions can be attributed to another. The deposition method consists in the deposition of various compounds of metals from solutions of their salts using precipitators. The precipitation product is metal hydroxides. The control of the pH and the temperature of the solution is possible to create optimal deposition conditions for obtaining nanomaterials, under which crystallization rates increase and highly dispersed hydroxide is formed. Then the product is calcined and, if necessary, restore. The resulting nanopowders of metals have a particle size from 10 to 150 nm. The shape of individual particles is usually close to spherical. However, by this method, varying the parameters of the deposition process, you can get a needle powder, scaly, incorrect form. The sol-gel method was originally designed to obtain iron powder. It combines the process of chemical cleaning with the recovery process and is based on the deposition of aqueous solutions Insoluble metal compounds in the form of a gel obtained using modifiers (polysaccharides), with their subsequent recovery. In particular, the PE content in the powder is 98.5 - 99.5%. Salts of iron can be used as raw materials, as well as metallurgical production: metal scrap or waste film solution. Through the use of secondary raw materials, the method provides the possibility of producing clean and cheap iron. This method can also be obtained by other classes of materials in nano-bearing: oxide ceramics, alloys, metals salts, etc. Restoration of oxides and other solid metal compounds is one of the most common and economical methods. Gases are used as reducing agents - hydrogen, carbon monoxide, converted natural gas, solid reducing agents - carbon (coke, soot), metals (sodium, potassium), metal hydrides. Source raw materials may be oxides, various chemical compounds Metals, ores and concentrates after appropriate preparation (enrichment, removal of impurities, etc.), waste and by-products of metallurgical production. The size and shape of the resulting powder is influenced by the composition and properties of the source material, reducing agent, as well as the temperature and recovery time. The essence of the method of chemical reduction of metals from solutions is to restore metal ions from aqueous solutions to their salts with various reducing agents: H2, CO, hydrazine, hypophosphite, formaldehyde, etc. In the method of gas-phase chemical reactions, nanomaterials are carried out due to chemical interaction in the atmosphere of vagalltechy vapor connections. Nanopoproops are also manufactured using thermal dissociation or pyrolysis processes. The decomposition is subjected to salts of low molecular weight organic acids: formates, oxalates, metal acetates, as well as carbonates and carbonyls of metals. The temperature interval of dissociation is 200 - 400 o C. The method of electrodeposition is to precipitate the metal powder from aqueous solutions of salts when passing direct current. Approximately 30 metals are obtained by the electrolysis method. They have high purity, since refining occurs during electrolysis. Metal precipitating on the cathode, depending on the electrolysis conditions, can be obtained as a powder or sponge, dendrites that are easy to mechanically grinding. Such powders are well pressed, which is important in the production of products. Nanomaterials can be carried out in biological systems. As it turned out, nature uses materials of nanoscalem millions of years. For example, in many cases, living systems (some bacteria, the simplest organisms and mammals) produce minerals with particles and microscopic structures in the nanometer range of the sizes. It was found that biological nanomaterials differ from others, since their properties were developed by an evolutionary way for a long time. In the biomineralization process, the mechanisms of fine biological control are operating, resulting in materials with clearly defined characteristics. This ensured a high level of optimizing their properties compared to many synthetic nanoscale materials. Live organisms can be used as a direct source of nanomaterials whose properties can be changed by varying the biological synthesis conditions or during processing after extraction. Nanomaterials obtained by biological methods can be the starting material for some standard methods of synthesis and processing nanomaterials, as well as in a number of process technologies. While still work in this area is a bit, but there are already a number of examples that show that there is a significant potential for future achievements in this direction. Currently, nanomaterials can be obtained from a number of biological objects, namely:
- 1) ferritines and associated proteins containing iron;
- 2) magnetotactic bacteria;
- 3) pseudocubs of some mollusks;
- 4) with the help of microorganisms by extracting some metals from natural compounds.
Ferritins are a class of proteins that provide for living organisms the ability to synthesize particles of hydroxides and oxyphosphates of iron nanometer size. It is also possible to obtain nanomeal with microorganisms. The processes of using microorganisms can be divided into three groups. The first group includes the processes that have been used in industry. This includes: bacterial leaching of copper from sulphide materials, bacterial leaching of uranium from ore, separation of mysteria impurities from tin and gold concentrates. In some countries, up to 5% of copper, a large amount of uranium and zinc is obtained by microbiological methods. The second group includes microbiological processes, well-studied in laboratory, but not brought to industrial use. This includes the processes of extracting manganese, bismuth, lead, Germany from poor carbonate ores. As it turned out, with the help of microorganisms, you can open fine-lined gold in arsenopyric concentrates. Gold, which relates to difficult to oxidized metals, under the influence of some bacteria forms compounds, and due to this can be extracted from ores. The third group includes theoretically possible processes requiring additional study. These are the processes of producing nickel, molybdenum, titanium, thallium. It is believed that under certain conditions, the use of microorganisms can be used in the processing of poor ores, dumps, "tailings" of processing factories, slags.
Send your good work in the knowledge base is simple. Use the form below
Students, graduate students, young scientists who use the knowledge base in their studies and work will be very grateful to you.
Posted on http://www.allbest.ru/
Nanotechnology is a region of fundamental and applied science and technology, dealing with a combination of theoretical substantiation, practical methods of research, analysis and synthesis, as well as methods of production and use of products with a predetermined atomic structure by means of controlled manipulation by individual atoms and molecules.
The basis of all Nano technologies is the ability of the four-grained elements (most often carbon) to form polyatomic, and then multi-molecular structures. Such structures most often have specific (depending on the composition, forms of the resulting molecule and its other parameters) properties that do not have any other known compounds, which makes them such interesting for science and opens up huge areas for the use of nanoolecules and overall nanotechnology. Nanotechnology Technique Material
For example, it turned out that nanoparticles of some materials have very good catalytic and adsorption properties. Other materials show amazing optical properties, for example, ultra-thin films of organic materials are used to produce solar panels.
In turn, the ability of tetravalent elements, for example, carbon, to form four bonds with other atoms is due from the point of view of physics by the presence of four valence electrons in the external energy level.
Of course, it should be said that such an explanation does not quite reveal the question and is more chemical, and not physical. But if you can drop further, you can see that the basis is physical phenomenonwhich explains the formation of ties between atoms.
Also note, the current description of the chemical bond is carried out on the basis of the quantum mechanics that is a section of physics. Chemical bond is determined by the interaction between charged particles (nuclei and electrons). Such interaction is called electromagnetic.
Methods for obtaining nanomaterials are divided into mechanical, physical, chemical and biological. Those. This classification is based on the nature of the synthesis of nanomaterials. The basis of mechanical methods of obtaining is the impact of large deforming loads: friction, pressure, pressing, vibration, cavitation processes, etc. Physical methods of obtaining are based on physical transformations: evaporation, condensation, sublimation, sharp cooling or heating, spraying melt, etc. (For completeness of classification and for reference), the methods of which are the main dispersing step of which are: electrolysis, recovery, thermal decomposition. Biological methods of obtaining are based on the use of biochemical processes occurring in protein bodies.
Mechanical methods Mechanical effects when grinding materials is impulse, i.e. The occurrence of the field of stress and its subsequent relaxation occurs not during the entire time of the residence of particles in the reactor, but only at the time of the collision of the particles and in a short time after it. The mechanical impact is also local, as it does not occur in the entire mass of the solid substance, and where the voltage field occurs and then relaxes. Due to the impulse and locality in small areas of the material for a short time, large loads are focused. This leads to the emergence of defects, stresses, shear strips, deformations, cracks. As a result, the grinding of the substance occurs, mass transfer is accelerated and mixing components, the chemical interaction of solid reagents is activated. As a result of mechanical abrasion and mechanical fusion, a higher mutual solubility of certain elements in solid state can be achieved than possible in equilibrium conditions. Grinding is carried out in ball, planetary, vibration, vortex, gyroscopic, inkjet mills, attributes. Grinding in these devices occurs as a result of shocks and abrasion. The method of mechanical grinding method is a mechanochemical method. With a thin grinding of a mixture of various components between them, interaction is accelerated. In addition, there may be chemical reactions that, when contacting, not accompanied by grinding, do not occur at all at such temperatures. These reactions are called mechanochemical. In order to form a nanostructure in volumetric materials using special mechanical deformation schemes, which allow you to achieve large distortion of the structure of the samples at relatively low temperatures. The following methods include intensive plastic deformation:
High pressure tapping;
Equational angular pressing (RKU-pressing);
Comprehensive forging method;
Equational angular hood (RCU-hood);
The "hourglass" method;
Intensive friction method with sliding.
Currently, most of the results are obtained by the first two methods. Recently, methods for obtaining nanomaterials using mechanical exposure to various environments are being developed. These methods include cavitation-hydrodynamic, vibration methods, a shock wave method, ultrasound grinding and detonation synthesis.
The cavitation and hydrodynamic method is used to obtain nanopowders suspensions in various dispersion media. Cavitation - from Lat. The words "emptiness" - formation in liquid cavities (cavitation bubbles or cavities) filled with gas, ferry or mixture thereof. During the process, cavitation effects caused by the formation and destruction of the vapor-gas microbubbles in the liquid for 10-3 - 10-5 s at pressures of the order of 100-1000 MPa, they lead to warming not only liquids, but also solid tel. This impact causes the grinding of solid particles.
The grinding of ultrasound is also based on the precipitation of cavitation strikes. The vibration method for obtaining nanomaterials is based on the resonant nature of effects and phenomena, which provide minimal energy consumption during processes and a high degree of homogenization of multiphase media. The principle of operation is that any vessel is subjected to a vibrational effect with a certain frequency and amplitude.
Almaz nanoparticles can be obtained by detonation synthesis. The method uses the energy of the explosion, while the pressure is achieved in hundreds of thousands of atmospheres and temperatures up to several thousand degrees. These conditions correspond to the area of \u200b\u200bthermodynamic stability of the diamond phase. Physical methods of obtaining materials include spraying methods, condensation evaporation processes, vacuum sublimation technologies, techniques in solid state.
The method of spraying the melt stream with liquid or gas is that the thin jet of the liquid material is supplied to the chamber, where it is broken into small drops with a stream of compressed inert gas or a fluid jet. As gases in this method use argon or nitrogen; As liquids - water, alcohols, acetone, acetaldehyde. The formation of nanostructures is possible by managing a liquid state or spinning. The method consists in obtaining thin ribbons using a quick (at least 106 k / s) melt cooling on the surface of the rotating disk or drum.
Physical methods. Methods of evaporation-condensation are based on the preparation of powders as a result of the steam transition - a solid body or steam - a solid body in a gas volume or on a cooled surface.
The essence of the method is that the starting material evaporates through intense heating, and then cooled sharply. The heating of the evaporated material can be carried out in various ways: resistive, laser, plasma, electric arc, induction, ionic. The process of evaporation condensation can be carried out in vacuo or neutral gas medium. The electric explosion explosion is carried out in argon or helium at a pressure of 0.1 - 60 MPa. In this method, thin metal wires with a diameter of 0.1 - 1 mm are placed in the chamber and the current of high force is impulse.
The duration of the pulse is 10-5 - 10-7 s, the current density is 104 - 106 A / mm2. At the same time, the wires are instantly warmed up and explode. The formation of particles occurs in a free flight. Vacuum sublimation technology of obtaining nanomaterials includes three main stages. In the first stage, the original solution of the treated substance or several substances is prepared. The second stage - the freezing of the solution - aims to fix the uniform spatial distribution of components inherent in the fluid to obtain the minimum possible size of crystallites in the solid phase. The third stage is the removal of solvent crystallites from a frozen solution by sublimation.
There are a number of methods for obtaining nanomaterials in which dispersion is carried out in a solid matter without changing the aggregate state. Differences from the methods of obtaining massive nanomaterials is a method of controlled crystallization from an amorphous state. The method involves obtaining an amorphous material by hardening from a liquid state, and then in conditions of controlled heating, a crystallization of the substance is carried out. Currently, the most common method of obtaining carbon nanotubes is the method of thermal spraying of graphite electrodes in the plasma of arc discharge.
The synthesis process is carried out in a chamber filled with high pressure helium. When the plasma is burning, there is an intense thermal evaporation of the anode, while the precipitate is formed on the end surface of the cathode, in which carbon nanotubes are formed. The resulting numerous nanotubes have a length of about 40 microns. They increase on the cathode perpendicular to the flat surface of its end and are collected in orindric bundles with a diameter of about 50 microns.
Nanotube beams regularly cover the surface of the cathode, forming a cellular structure. It can be found, looking at the precipitate on the cathode with an e-shown eye. The space between the nanotube beams is filled with a mixture of disordered nanoparticles and single nanotubes. The content of nanotubes in the carbon sediment (deposit) can approach 60%.
According to a small study conducted by me on modern technologies that are introduced in the manufacture of clothing, I can say that some technologies are already actively used when creating materials for clothing and shoes, but as for biocoes and nanotechnology, as long as information about such experiments, such as Olivia ONG , very little and it is rare enough in the network. I found about 10 examples of mentioning the use of nanomaterials in creating clothes.
... Unusual clothing developed by the Japanese research team Life Beans ...
... or Krichevsky Herman Essayevich, Professor, Doctor of Technical Sciences, Honored Worker of the Russian Federation, UNESCO Expert, Academician RIA and Mia, Laureate of Mr. MSR tells in the article for Nanonewsnet.ru on their experience in the introduction of nanotechnology on textile production ...
... Chinese scientists have created a nodule, which is cleaned by the influence of solar radiation ...
... Portugal is developing new materials and devices that are the last word in innovation within the framework of the European Research project Dephotex ...
And several other references to other projects.
Unfortunately, despite some successes in the field of bio and nanotechnology and even specifically the area of \u200b\u200bclothing, the products obtained remains exormably expensive for both the manufacturer and for the buyer therefore nanotechnology clothing is not yet ready to be made in larger quantities. Today, this area is actively developing and remains a promising direction in the field of nanotechnology.
According to the forecasts of some scientists, the importance of accessibility high technologies In the future, it will be achieved through the search for rational methods and technologies for obtaining various nanomaterials and will ultimately lead to the widespread replacement of ordinary materials on those who were obtained using high technologies.
The leader in the study of methods for obtaining nanomaterials is NSTU and TPU in particular, the Department of Biotechnology on the basis of the Institute of High Technology Physics.
Posted on Allbest.ru.
...Similar documents
General On the methods of obtaining nanoparticles. The main processes of cryochemical nanotechnology. Preparation and dispersion of solutions. Biochemical methods for obtaining nanomaterials. Freezing of liquid droplets. Supervisual gas outflow from nozzle.
course work, added 11/21/2010
Study of the features of volumetric nanostructured materials. History of Nanotechnology. Causes of widespread interest in nanotechnology and nanomaterials. Methods of obtaining nanopowders. Plasmochemical and cryochemical synthesis. Products of cryotechnology.
presentation, added 12/25/2015
Fullerites as a crystal from large carbon molecules CN Fullerenes. Acquaintance with the main features of nanocrystalline materials, advantage analysis: high viscosity, increased wear resistance. Characteristics of the mechanical properties of nanomaterials.
abstract, added 05/20/2014
A group of quantitative chemical analysis methods based on the use of electrolysis (electrochemical methods of analysis). Features of the electrogrammetric method, its essence and application. Main equipment, internal electrolysis method.
abstract, added 15.11.2014
Nanocatalysis as a rapidly developing area of \u200b\u200bscience, which includes the use of nanomaterials as catalysts for various processes of catalysis. Features of the production of nanoscale catalysts with 100% selectivity and high activity.
abstract, added 01/06/2014
The effect of mechanical activation on the geometric parameters of dispersed materials. The main equipment used for sedimentation analysis of materials. Development of the installation for research materials, technical and economic rationale for this process.
thesis, added 04/16/2014
The concept and appointment of chemical methods analysis methods, the procedure for their conduct and evaluation of efficiency. Classification and varieties of these methods, types of chemical reactions conducted. Forecasting and calculation of the physicochemical properties of different materials.
lecture, added 08.05.2010
Theoretical aspects methods. Essence of testing materials for resistance to microscopic mushrooms and to bacteria. Features of measuring the bioluminescence intensity and toxicity index. The main parameters for estimating the bioscistance of building materials.
abstract, added 01/13/2015
One of the most promising and promising development directions modern science is nanotechnology. The study of nanocomposites from ceramics and polymers, nanocomposites containing metals or semiconductors. Nanotechnology capabilities.
abstract, added 01/26/2011
Studying chemical methods for obtaining powders: restoration of oxides and salts of metals with solid or gaseous reducing agents, dissociation of carbonyls and unstable compounds, metalothermia. Removing iron from used automotive tires.
Methods for obtaining nanocrystalline materials:
1. Gas phase synthesis of nanoparticles.
2. Plasmochemical synthesis.
3. Deposition from colloidal solutions.
4. Mechanical methods.
5. Detonation synthesis of nanoparticles.
6. High-speed cooling.
7. Methods for obtaining large degrees of deformation.
8. Crystallization of glassy structures.
9. Thermal decomposition and recovery.
Deposition from the gas and liquid phase. Fast solidification from melt. Intensive plastic deformations. Recrystallization from amorphous state. Compact nanomaterials. Advantages and disadvantages of various techniques.
Gas phase synthesis of nanoparticles
Separate nanoparticles in gas-phase synthesis are obtained in the process of evaporation and subsequent condensation of the material in the medium of inert gas.
At the evaporation stage, heating of the evaporated material of high frequency currents, an electric arc discharge, laser or electron beam, current passing, as well as radiation heating can be used.
The condensation of the formed pair occurs when it collide with molecules of inert gas, with the walls of the reaction chamber, as well as due to adiabatic expansion when entering a large volume or use of the nozzle of Laval
Gas phase synthesis allows you to get particles of 2 to several hundred nanometers
The shape of nanoclusters with a size of less than 20 nm is close to spherical, with large sizes acquires cut. The distribution of nanoclusters in size obeys a logarithmically normal law.
For collecting powders, special filters and centrifugal deposition are used; In some cases, the capture of liquid film is applied.
The size and location of the condensation region depends on the pressure of the inert gas in the chamber. With a large pressure, the condensation area is concentrated near the evaporator, with a decrease in pressure, the outer limit is outside the reaction chamber.
The use of heavier inert gas molecules leads to an increase in nanoclusters.
When forming inside the volume of the chamber, nanoclusters of a rounded form are formed, and on the walls, as a rule, nanoclusters with cutting. Under the same evaporation conditions and condensation, materials having a higher melting point form smaller particles.
If there is more than one element in the chamber, it is possible to synthesize the compounds, and to give particles with different shapes.
One of the plants for the levitational source, gas-phase synthesis (Fig.) Is a column, in the upper part of which evaporates from the surface of a liquid drop at the end of the wire.

Fig. 1.1. Levitational Nursing Installation for obtaining highly dispersed metal powders: 1 - evaporator, 2 - drop, 3 inductor, 4 - aerosol, 5 - refrigerator, 6 - filter, 7 - container, 8 -PCOS, 9 - Wire supply mechanism
Wire melting is achieved by high-frequency electromagnetic field Inductor. The sprayed material is enjoyed by the stream of inert gas into the middle part of the column, which is the reaction chamber. Faced with molecules of inert gas, as well as with the walls of the chamber, the evaporated atoms are inhibited with the formation of nanoclusters. An increase in the gas flow rate reduces the average particle size and narrows the particle size distribution
Nanoclusters, passing the filter, collected in the container.
At the installation, powders are obtained with a size of 5 to 200 nm.
Smaller nanoclusters can be obtained using a magnetic or time mass channel.
The principle of operation of the magnetic mass spectrometer is based on the force of Lorentz F, acting on a positive charge q moving at a velocity V in a magnetic field with induction B, perpendicular to the magnetic power lines.
It is known that in this case the mass particle M with charge Q, will move around the circle of the radius R
By determining, thus, the mass of the particle M can estimate its transverse size D, knowing its density R and the form
The mass spectrometer (Fig.) Consists of a source of ionized particles, a mass channel and a collector of particles.

Fig. Massanitizer
The source takes place:
ionization of particles due to bombardment by electron beam 1,
acceleration of particles with an electric field with a potential difference U to give a constant speed V, which is based on the equality of potential (U * Q) and kinetic (MV 2/2) particle energies.
focusing with magnetic lenses 2.
The particles with the same charge q, flying in vacuo at a rate of V, fall into the magnetic field B of the Multivatorizer, where their selection occurs depending on the mass M. Leaving the Mass Namer, particles reach the detector in the ion collectors. The collector's gap includes particles with a certain mass m. In order to register and collect particles of various sizes, you can change the values \u200b\u200bof B or U, controlling the trajectory of particles.
At the time of therent analyzer, the ionized metal pairs are passed through a cell under a pressure under a pressure of about 1000-1500 Pa, accelerating to a certain velocity V in an electric field with a potential difference U, then admitted to the vacuum chamber (~ 10 5 PA), where the cluster size is set through it The weight of the time of flight in the braking electrical field E.

For diamond synthesis, a gas phase is created, for example, CO 2, which is dereoned by carbon content. As a result, on the border of the section, the solid body-gas occurs to the condensation of carbon from the gas phase and the formation of diamond germs. The synthesis of the gas phase is conducted in the metastable for diamond conditions: at a pressure of several paging to several hundred GPa and a temperature of 870-1070 K. due to the low growth rate (~ 100 nm / hour), nanoscale diamond particles can be obtained. The deposition of diamond nanoparticles found the greatest application To create diamond and diamond-like films and coatings ...
Silver, lithium and copper nanoparticles, besieged on the glass, were obtained by evaporation of metals in an inert atmosphere at a pressure of 0.01 - 0.13 Pa. Al 2 O 3, ZRO 2, Y 2 O 3 nanocrystalline oxides were evaporation of oxide blanks in a helium atmosphere, magnetron sputtering of zirconium in a mixture of argon and oxygen, controlled by the oxidation of yttrium nanocrystals.
One of the most effective methods of applying films in planar technology is the method of magnetron spraying of materials. This method is a kind of ion-plasma spraying. Spraying of the material in these systems occurs due to the bombardment of the target surface of the work gas ions. The spraying rate in the magnetron system is 50 - 100 times higher than a conventional ion-plasma spray. The high speed of spraying of the material in the magnetron spray system is determined by the high density of the ion current on the target. The high density of ionic current is achieved due to the localization of the plasma at the target surface with a strong transverse magnetic field.

Fig. 1.1. The diagram of the magnetron spray system:
1 - target; 2 - magnetic system; 3 - spray zone; 4 - magnetic power lines; 5 - stream of sprayed substance; 6 - substrate; 7 - substrate holder.
The diagram of the magnetron spray system is shown in Figure 1.1. The main elements of the system are target 1 and magnetic system 2. Magnetic power lines 4 are closed between the poles of the magnetic system. Between the target 1 and the substrate holder 7, an electric field is applied and an abnormal glowing discharge is excited. A closed magnetic field near the target surface localizes the discharge near this surface. Positive ions from the plasma of an abnormal intelligence discharge are accelerated by an electric field and bombard the target (cathode). Under the influence of ion bombardment, target spraying occurs. Electrons issued from the cathode under the action of ion bombardment fall into the area of \u200b\u200bcrossed electrical and magnetic fields and are trapped. The trajectories of electron movement in a trap are close to cycloidal. The ionization efficiency and plasma density in this area increases significantly. This leads to an increase in the concentration of ions in the target surface, an increase in the intensity of the ionic bombing of the target and to a significant increase in the target spraying rate.

Nanopowders nitrides transition metals Formed with electronically heating and subsequent evaporation in a nitrogen or ammonia atmosphere at a pressure of 130 Pa.
Nanoparticles of carbides, oxides and nitrides are also obtained by pulsed laser heating of metals in a rarefied atmosphere of gases-reagents: methane (carbides), oxygen (oxides), nitrogen or ammonia (nitrides). In an atmosphere of inert gas (non-AG) and a gas panel (O 2, N 2, NH 3, CH 4), mixtures of nanocluster oxides of various metals are formed, as well as oxidendridrid or carbide-bridge mixtures.
The composition and size of the nanoparticles is regulated by the pressure and composition of gases, the capacity of the laser pulse, the temperature difference between the evaporated target and the precipitation surface.
In the installation for obtaining ceramic nanopowers from metal organo precursors (Fig.) The evaporator is a tubular reactor in which the precursor (intermediate reaction product) is mixed with the carrier inert gas and decomposes. The forms of the continuous flow of clusters or nanoparticles falls from the reactor to the working chamber and condenses on a cold rotating cylinder. The formation of nanoclusters is ensured by a low concentration of the precursor in the inert gas, the rapid expansion and cooling of the gas flow when exiting the reactor to the working chamber, low pressure in the working chamber.

Rice .. Scheme of equipment for obtaining nanocrystalline ceramic powders by steam condensation (CVC) using metal-organic precursors as a source of condensed pair
Plasmochemical synthesis
At the first stage of plasma-chemical synthesis, the formation of active particles in arc, high-frequency and ultra-fast-frequency under the action of an electric arc, microwave fields in plasma reactors.
At the second stage, the nanoparticles are highlighted as a result of cooling.
Plasmochemical synthesis is used to obtain highly dispersed nitride powders, carbides, borides and oxides.
Plasmochemical synthesis It is advisable to expediently carry out a high cooling rate of the plasma flow in which condensation of the gas phase nanoparticles occurs; Due to which the particle generation size decreases, and the combination of particles in a collision is also suppressed.
As a raw material use chemical elements and their compounds, in particular, halides. In this case, the synthesis uses nitrogen, ammonia, hydrocarbon, argon low-temperature plasma arc, smoldering, highly or ultrahigh frequency discharges (40008000 K).
The resulting particles of plasma-chemical powders are single crystals and have dimensions from 10 to 200 nm and more. Disadvantages of plasma chemical synthesis (large particle size scatter, high impurity content) is compensated by a large process performance and a large variety of powders: (nitrides (titanium, zirconium, hafnium, vanadium, niobium, tantalum, boron, aluminum and silicon), carbides (titanium, niobium , tantalum, tungsten, boron and silicon), oxides (magnesium, yttria and aluminum).
Plasmochemical powders of metal carbides, boron and silicon are usually obtained by the interaction of chlorides of the corresponding elements with hydrogen and methane or other hydrocarbons in argon high-frequency or arc plasma; Nitrides are obtained by the interaction of chlorides with ammonia or a mixture of nitrogen and hydrogen in low-temperature BCCPLAZ. With the help of plasma chemical synthesis, multicomponent submicrocrystalline powders are also taken, which are mixtures of carbides and nitrides, nitrides and borides, nitrides of different elements, etc.
The synthesis of oxides in the plasma of the electric arc discharge is carried out by evaporation of the metal with the subsequent oxidation of the vapor or the oxidation of the metal particles in the oxygen-containing plasma.
The plasmochemical method is used to obtain metal powders. For example, submicrocrystalline copper powders with a particle size of less than 100 nm and a relatively narrow distribution of particles in size are obtained by the reduction of hydrogen chloride in argon electric arc plasma with a temperature of up to 1800 K.
Gas phase synthesis
Gas phase synthesis is one of the varieties of plasma chemical synthesis, in which heating, evaporation and the flow of gas-phase reactions of the original raw material is carried out by a laser beam.
Laser heating ensures the formation of homogeneous size and composition of nanoparticles.
Silicon particles with a diameter of 50 ± 20 nm are obtained from gaseous SIH 4 using CO 2 laser. The si powder of spherical powder had and consisted of several crystallites with a size of ~ 15 nm.
Si 3 N 4 silicon nitride powders were synthesized from gas mixture SIH 4 and ammonia NH 3. The resulting powder was amorphous, the grain of the powder had a spherical shape and an average size of 17 ± 4NM, and the distribution of the grain in size is narrower, which in the Si powder (for Si 3 N 4, the scattering boundaries in size from 10 to 25 NM). In contrast to the silicon nanopowder, Si 3 N 4 did not have an internal structure. For the synthesis of SIC silicon carbide, gas mixtures with methane or ethylene were used. The size of the grain in the resulting crystalline SIC powder ranged from 18 to 26 nm, the average size was 21 nm. The study showed that the size of nanocrystalline particles decreases with increasing intensity (power referred to unit area) laser radiation Due to the increase in temperature and speed of heating of gas stearters. The ignition of nanopowers, synthesized with the use of laser heating, is distinguished by a narrow distribution in size and a spherical shape.
Gas phase synthesis using laser radiation to create and maintain plasma in which a chemical reaction occurs, turned out to be an effective method for producing molecular clusters.
Molecular clusters occupy a very special place among substances having a nanostructure. The most famous among them are a new allotropic carbon modification along with graphite and diamond.
The method of plasma chemical gas phase synthesis was obtained by Ti 8 C 12 clusters. Helium was used as an inert gas; The reagents were hydrocarbons (methane, ethylene, acetylene, propylene and benzene) and titanium pairs; The pressure of the gas mixture in the reactor was 93 pa. To evaporate the rotating metal rod of titanium and the creation of an ionized beam of metal vapors was used focused radiation of the Ndladzer with a wavelength of 532 nm. In the mass spectra of the reaction products, a sharp peak was detected corresponding to the Ti 8 C 12 molecule. The high stability of the TI 8 C 12 cluster is, an imaginary, consequence of a special geometric and electronic structure inherent in such clusters.
The binders of the Ti 8 C 12 cluster are formed by a combination of 3D Ti and molecular orbitals with 2, and the filled level with the highest energy is located between the binders and the anti-binding states of titanium, which ensures the stability of the cluster.
Sol-gel technology
Salty (Losoli) - highly dispersed colloid systems with a liquid dispersion medium. Particles of the dispersed phase of sol, together with the surrounding solvate sheath of molecules (ions) of the dispersion medium, called. Micelles.

They are free and independently of each other participate in Brownian motion and evenly fill the entire volume of the dispersion medium. The particle size of Liosol usually lies within 10 -7 -10 -5 cm. Sague with water dispersion medium. Hydrosols, with org. Wednesday - OrganoFores. The evils are divided into lyophilic and lyophobic (strongly and weakly interacting D.F. with D.S.). The structure of micelles, for example, Agbr hydrosol, stabilized KVR, can be recorded using chemical. Symbols: (T PVR - (P - X) K +) HC +
Gels - gel-dispersed systems with a liquid dispersion medium, in which the particles of the dispersed phase form spaces. Structural grid. They are solid-shaped ("centers") of the body, capable of preserving the shape with elasticity (elasticity) and plasticity. Typical gels have a coagulation structure, i.e. The particles of the dispersed phase are connected in the contact places by the forces of the intermol. Interaction directly or through a thin layer of dispersion medium. They are characterized by the ability to isothermich. The conditions spontaneously restore their structure after fur. destruction.
SOL-GEL TECHNOLOGY (GEL TECHNOLOGY) - MATERIAL TECHNOLOGY MATERIALS MAINTENANCE TO OBJECTIVE OF COLOR AND PURCHASE IN GEL. Sol-gel technologies are used in the production of inorganic sorbents, catalysts and carriers of catalysts, synthetic zeolites, binding inorganic substances, ceramics with special thermophysical, optical, magnetic and electrical properties, glass, glass-ceramic, fibers, et al. In the first stage of the sol-gel technology Forming chemical composition The product that is obtained in the form of a highly dispersed colloidal solution is sol. The particle size of the dispersed phase in a stable ash 1 - 1000 nm. An increase in the concentration of the dispersed phase leads to the appearance of coagulation contacts between particles and the beginning of structuring - gelation (second stage of sol-gel technology). To increase the stability of structures and control processes of structural formation, affect the strength of contacts by modifying the surface of the particles with additives of surfactants or by creating a spatial structure of a high-molecular organic polymer. Highly concentrated dispersed systems are used in the production of inorganic binders and various pastes. Such systems have high plasticity. Coagulation forces are capable not only to preserve the shape of the gel, which is important when forming products, but to cause a gradual seal of the gel, accompanied by the separation of the dispersed phase from the gel, a decrease in its volume, an increase in density and strength. When removing the dispersion medium (the third stage of the process), strong phase contacts appear. When drying the gel turns into a solid tight-rope body (xerogel). In the drying process, a noticeable seal of the gel can occur and the change in its structure. Drying methods have been developed that reduce this effect and ensuring materials with high open porosity. Due to the high dispersion of xerogels (particle size of 10-100 nm) by molding and sintering, durable, dense products with a certain geometric shape made of refractory materials are produced.
Deposition from colloidal solutions
Getting ash.
The development of methods for the synthesis of highly dispersed colloidal systems began in the middle of the XIX century, after receiving the pharade of stable gold ash (2-50 nm) with the restoration of the diluted salt of gold with yellow phosphorus.
AUCL 3 + 3H 2 O + P ® AU + P (OH) 3 + 3HCl.
Later, classical methods of synthesis of monodisperse gold solishes were developed with a given degree of dispersion of gold with hydrogen peroxide and formaldehyde.
2 HAUCL 4 + 3H 2 O 2 ® 2 AU + 8HCl + 3O 2,
2 HAUCL 4 + 3HCHO + 11KOH ® 2AU + 3HCOOK + 8KCl + 8H 2 O
The process takes place in two stages. First, the embryos of the new phase are formed, and then in the ash, weak superstruction is created, in which the formation of new embryos is no longer occurs, but only their growth is. In this way, you can get yellow (D ~ 20 nm), red (D ~ 40 nm) and blue (D ~ 100 nm) gold evaluation.
To obtain oxide and hydroxide hydroxides, hydrolysis reactions of inorganic metals or metal avlexides are used. For example, the hydroxide sol of iron can be obtained by reaction:
FECL 3 + 3H 2 O + T (90 - 100ºC) "Fe (OH) 3 + 3HCl
The presence of a large excess energy associated with a highly developed interfacial surface of the section in ultrafaceous systems of a large excess of the interfacial surface of the section is promoting the processes of aggregation of colloidal particles. To obtain particles of a given dispersion, it is necessary to stop the growth of particles in time. To this end, the surface of the dispersed phase particles inhibit due to the formation of the protective layer from the surfactant or due to the formation of complex compounds on it.
The formation of micellar systems .
To obtain micellar systems, it is necessary to use surfactants - organic substances (synthetic and natural), which have limited solubility in water and capable of adsorbing on the surface of the phase separation, reducing the interfacial tension. These substances have a distillation: a molecule or ion of Pav contains a hydrophobic part and a polar group of one or another nature. The hydrophobic part represents a hydrocarbon radical (C n h 2 n + 1, with n h 2 n - 1, with n h 2 n + 1, C 6 H 4 and others), containing from 8 to 18 carbon atoms. Water at room temperature is a structured liquid, has a dipped order (R< 0,8 нм). При растворении ПАВ происходит дальнейшее структурирование молекул воды вокруг неполярных углеводородных радикалов ПАВ, что приводит к уменьшению энтропии системы. Поскольку система стремится к максимуму энтропии, то при достижении определённой концентрации, называемой критической концентрацией мицеллообразования (ККМ), молекулы или ионы ПАВ начинают самопроизвольно мицелл. Образование мицелл фиксируется обычно по изменению какого либо физического свойства раствора ПАВ (например, поверхностного натяжения, электропроводности, плотности, вязкости, светорассеяния и т. д.) в зависимости от концентрации ПАВ. При концентрациях, близких к ККМ, мицеллы представляют собой примерно сферические образования, в которых полярные группы контактируют с водой, а гидрофобные радикалы находятся внутри, образуя неполярное ядро. Молекулы или ионы, входящие в состав мицеллы, находятся в динамическом равновесии с объёмом раствора. Это является одной из причин «шероховатости» внешней поверхности мицелл.
At concentrations of surfactants, large KKM, the formation of several types of micelles (Fig) differing in form: spherical, cylindrical, hexagonally packaged, lamellar. Thus, micelles can be considered as one-dimensional, two-dimensional and volumetric nanoobjects. Depending on the nature of the surge of the aggregation number ( n.) They may vary from dozens to several hundred, while the dimensions of the micelle will change.
Paving molecules insoluble in water with a long hydrocarbon radical and a weak polar group can be dissolved in non-polar liquid phases. In this case, at a certain concentration of surfactants, a micelles are also observed, which is due to specific interactions between the Pavar Polar Groups. Such micelles are called back. The form of reverse micelles depends on the concentration of surfactants and may be different.

Figure 1. Structures arising in solutions of surfactants.
1 - Monomers, 2 - Micelles, 3 - cylindrical micelles, 4 - hexagonally packed cylindrical micelles, 5 - laminar micelles, 6 - hexagonally packed water drops in the reverse micellar system.
Education of microemulsions
Microemulsions are thermodynamically stable isotropic dispersions of two unsuccessful liquids. When mixing such liquids of a drop of one of them, stabilized by the interfacial film surfactant and sopav, which low-molecular weight alcohols are used, are distributed to another. Microemulsions relate to lyophilic dispersed systems and can be obtained either by spontaneous dispersion of two unsuccessful liquids as a result of a strong decrease in interfacial tension, or in solubilization process, as noted above. Thermodynamic stability of microemulsion systems is due to low interfacial tension, which can be 10 - 5 mJ. M - 2 for ion surfactants and 10 - 4 mJ. M - 2 for non-ionic surfactants. Depending on which phase is dispersed, and which continuous, microemulsion can be straight - oil in water (m / c) - or reverse - water in oil (in / m). The term "oil" means non-polar organic fluid. In both cases, the dispersed phase consists of droplets, the size of which does not exceed 100 nm.
As a rule, microemulsions are multicomponent systems consisting of various structures (bilayer, cylindrical, spherical micelles). In the process of micelle formation, in addition to liquid isotropic micellar phases, optically anisotropic micellar phases are formed, for example, layered smectic and hexagonal phases consisting of rod-shaped units of endless length, that is, microemulsions have an internal microstructure, which is currently intensively studied by various methods. In the case when the water and oil content in the system is comparable, the formation of biscontinual systems is possible.
The properties of microemulsions are largely determined by the size and form of particles of the dispersed phase, as well as the rheological properties of interfacial adsorption layers formed by surfactants. Since microemulsions have great mobility and a large surface of the partition between phases, they can serve as a universal environment for many chemical syntheses, including to obtain solid nanoparticles.
Formation of solid particles in microemulsions
In the microemulsion system, the particle of the dispersed phase is constantly facing, coalesce and destroy again, which leads to a continuous exchange of their contents. The process of a collision of the drops depends on the diffusion of drops in the oil phase (for the reverse microemulsion system), while the exchange process is determined by the interaction of the adsorption layers of the surfactant and the flexibility of the interfacial surface (the latter circumstance is extremely important when conducting chemical reactions in such systems)

Fig. The reaction scheme flowing in the reverse microemulsion system.
Reverse microemulsion systems are often used to obtain solid nanoparticles. For this purpose, two identical microemulsion systems in / m are mixed, the aqueous phases of which contain substances A and B, which have a work-soluble compound of C during the chemical reaction, during coalescence of the drops in them as a result of the metabolism, a new connection is formed (Fig.). The dimensions of the particles of the new phase will be limited to the size of the polar phase drops.
Metal nanoparticles can also be obtained when introduced into a microemulsion containing a metal salt, a reducing agent (for example, hydrogen or hydrazine) or when gas passes (for example, CO or H 2 S) through the emulsion. It is in this way (the restoration of the salt of the corresponding metal or hydrazine) was first obtained by monodisperse metal particles PT, PD, RH and IR (with a particle size of 3 - 5 nm). A similar method was used to synthesize bimetallic platinum and palladium nanoparticles.
Currently, the deposition reactions in microemulsion systems are widely used to synthesize metal nanoparticles, semiconductors, carbonates and barium sulfates, calcium, strontium of SiO 2 monodisperse particles, high-temperature ceramics.
Despite the fact that the mechanism of formation of nanoparticles is finally not established, a number of factors affecting the reaction flow can be distinguished. The effect of the dispersed phase also has the influence of the nature of the surfactant, which are stabilizers of a microemulsion system. However, in all cases, the size of the nanoparticles formed in the reaction processes is controlled by the size of the droplets of the initial emulsion.
It should also be noted the use of microemulsion systems to obtain organic compoundsWhat is important when creating new dosage forms
Most studies in this area refers to the synthesis of spherical nanoparticles. At the same time, great scientific and practical interest is the preparation of asymmetric particles (threads, discs, ellipsoids) and precise control over their form. Conducting synthesis in reverse microemulsion systems made it possible to obtain nanofires Baco 3 as well as asymmetric nanoparticles of various substances with unusual magnetic properties.
Of great interest is the synthesis of nanocomposites consisting of particles of one material (particle size 50 - 100 nm) coated with a thin layer of another material.
Obtaining mono- and polymolecular layers.
Surfactants are capable of forming monomolecular films on various surfaces of the phase separation: water - air; solid body - liquid; Liquid - liquid. Such films can be considered as two-dimensional nanosystems. The monolayers of the surfactant on the surface of the water - air were first investigated by LangMyur, who developed an experimental method of studying such films (Langmur scales).
Monomolecular films on the surface of the liquid can be located in various states: gaseous, liquid and solid. These states are characterized different energy interactions between surfactant molecules.
Under certain conditions (pH, temperature) on the surface of the water section - air spontaneously formed structures with high degree The order in which the surfactant molecules (or ions) are arranged in such a way that the polar group is in solution, and the hydrocarbon radical is oriented into the air at a low angle to the interfacial surface. Self-organization processes in the film are due to the presence of dilution in the surfactant molecules and can be analyzed from the point of view of the interaction of the polar group with an aqueous substrate and the interaction of hydrocarbon radicals.
Significant interest is chemical reactions flowing in monomolecular films. By changing surface pressure, you can control the orientation of molecules in the surface layer and thereby specifically affect the flow of reactions. Thus, Langmuir film - Brojett is used to obtain solid solid nanoparticles of different nature directly in the process of chemical reaction or photochemical restoration of metals salts. Such processes occur in biological systems.
The films deposited on the surface of solid bodies can form both mono- and polis. For example, if a glass plate, located vertically, pull out of the water through the monolayer stearate barium, located on the surface of the water, the plate is covered with a layer of surfactant, in which hydrocarbon radicals are oriented outward. As a result, the surface of such a plate becomes hydrophobic. It can be applied to it the next layer. Hydrophilic or hydrophobic surfaces can be obtained by sequential application of the layers. The films constructed from equally oriented layers are called xplots, and from oppositely oriented - yarnings. Thus, polyolate coatings can be obtained, the thickness of which lies within the nanometer sizes.
The structure and form of ultrafine particles.
Issues relating to the mechanisms for the formation and structure of nanoscale particles are among the most important and fundamental issues of colloid chemistry. Indeed, ultradisperse particles are a kind of " elementary particles»Colloid chemistry. The transition from a simple qualitative determination of the very concept of dispersed particles to determine their quantitative parameters and ratios requires detailed clarification of the structure of ultrafine particles in various colloidal systems - ash, micellar solutions, microemulsions, gels, and so on.
The early concept of the structure of solid ultrafine particles was based on the assumption that their structure is similar to the structure of the macrophase of the same substance. However, further studying the process of nucleation and growth of the new phase showed that, depending on the crystallization conditions (the magnitude of the suspension or supercooling, the presence of impurities and a number of other reasons) from solutions can be formed both amorphous and crystalline ultrafine particles. Even Weimarne found that the shape of the formidized during crystallization from a solution of particles of BASO 4 depends on the degree of suction of the solution. So, they obtained highly dispersed evils, flake structures, well-cut microcrystals and a needle-like crystals. The temperature at which the synthesis of nanoparticles is carried out important role. For example, titanium dioxide nanoparticles obtained by zolgel by the method at low temperatures have the type of rods, and at high - bipyramidal crystals. Another confirmation of the diversity of the forms of nanoparticles is the formation of dendrites in crystallization from melts and solutions.
The variety of forms is due to the fact that the processes of the formation of a new phase (the processes of self-organization) proceed in purely non-equilibrium conditions, and the degree of perfection of the structure depends on how much the conditions for the crystallization are deviated from the equilibrium. For example, in the synthesis of a diamond of a dense gas phase and plasma, a more advanced structure is formed in more non-equilibrium conditions.
A strong effect on the crystallization process can be provided by surfactants present in solution. Depending on nature and concentration, they can change the rate of formation and growth of the nucleus of the new phase, the distribution of nanoparticles in size, as well as the form of crystals. All these effects are associated with the electoral adsorption of molecules or ions surfactants on various facilities of the resulting crystals and, as a result, with a slowdown in the growth of some faces compared to others. In addition, the nature of the surfactant affects the polymorphism of the formed compounds.
An important feature of crystallization processes leading to the formation of nanoparticles is that their form cannot be described by the methods of ordinary geometry. To describe such systems, fractal geometry is attracted, since with strong deviations from equilibrium, and therefore high values driving force The crystallization process, the unstability of the border of the phase partition leads, as a rule, to the formation of fractal structures.
Interesting, the results of works are presented, in which it is shown that with co-crystallization of ammonium halides and cesium iodide from highly healing vapors, highly dispersed primary single crystals are formed. Due to the developed interfacial surface, the resulting dispersed system has a large excess energy, therefore, aggregation processes are processed, accompanied by the capture of the initial monocrystalline particles of approximately equal dimensions. As a result of such aggregation, pseudomonocrystals are formed.
Fulleans are obtained by various methods, among which the arc method is common, production in flame, with laser heating, when evaporation of graphite, focused solar radiation, as well as chemical synthesis.
The most effective way to obtain fulleries is thermal spraying of the graphite electrode in the plasma of the arc discharge, Gelia burning in the atmosphere. There is an electric arc between two graphite electrodes, in which the anode is evaporated. On the walls of the reactor, a soot is deposited containing from 1 to 40% (depending on the geometric and technological parameters) of fullerenes. For the extraction of fullerenes from fullerene-containing soot, separation and purification, liquid extraction and column chromatography are used. Performance is no more than 10% of the weight of the original graphite soot, while in the final product, the ratio of 60: from 70 is 90: 10. To date, all fullerenes presented on the market are obtained by this method. The disadvantages of the method include the complexity of the discharge, cleaning and separation of various fullerenes from carbon soot, the low yield of fullerenes, and, as a result, their high cost.
The most common methods of nanotube synthesis are an electric arc discharge, laser ablation and chemical precipitation from the gas phase.
Using electric arc discharge The intensive thermal evaporation of the graphite anode occurs, and the sediment (~ 90% of the anode mass) is formed on the end surface of the cathode) of a length of about 40 microns. Bunches of nanotubes in a sediment on the cathode are visible even with a naked eye. The space between the beams is filled with a mixture of disordered nanoparticles and single nanotubes. The content of nanotubes in the carbon sediment may reach up to 60%, and the length of the resulting single-axis nanotubes is up to several micrometers at a small diameter (1-5 nm).
The disadvantages of the method include technological difficulties associated with the implementation of the multi-stage cleaning of the product from the particulate inclusions and other impurities. The output of single carbon nanotubes does not exceed 20-40%. Hasive amount of control parameters (voltage, strength and density of current, plasma temperature, general pressure in the system, properties and feed rate of inert gas, the size of the reaction chamber, the duration of the synthesis, the presence and geometry of cooling devices, the nature and purity of the material of the electrodes, the ratio of their geometric sizes , as well as a number of other parameters that are difficult to give a quantitative assessment, for example, the cooling speed of carbon vapors) significantly complicates the process regulation, the hardware design of the synthesis settings and prevents them from reproducing industrial applications. It also interferes with the modeling of arc synthesis of carbon nanotubes.
For laser ablation There is evaporation of a graphite target in a high-temperature reactor with subsequent condensation, while the yield of the product reaches 70%. With the help of this method, it is preferably one-way carbon nanotubes with a controlled diameter. Despite the high cost of the material obtained, laser ablation technology can be scaled to an industrial level, so it is important to consider how to exclude the risk of nanotubes into the atmosphere of the working area. The latter is possible with the full automation of the processes and the exclusion of manual labor at the packaging phase of products.
Chemical precipitation from the gas phase It occurs on the substrate with a layer of catalyst from metal particles (most often nickel, cobalt, iron or mixtures thereof). To initiate the growth of nanotubes to the reactor, two types of gases are introduced: technological gas (for example, ammonia, nitrogen, hydrogen) and carbon-containing gas (acitylene, ethylene, ethanol, methane). Nanotubes begin to grow on particles of metal catalysts. This method is most promoted on an industrial scale due to lower cost, relative simplicity and controlling the growth of nanotubes with a catalyst.
A detailed analysis of the products obtained by chemical deposition in the gas phase showed the presence of at least 15 aromatic hydrocarbons, including 4 toxic polycycle carbon compounds were detected. The most harmful in the composition of by-products of production was recognized by polycyclic benzapine - widely known carcinogen. Other impurities are a threat to the ozone layer of the planet.
Several Russian companies have already begun production of carbon nanotubes. Thus, the Scientific and Technical Center "Pomegranate" (Moscow region) has developed by its own pilot installation of the synthesis of carbon nanomaterials by the method of chemical precipitation with a capacity of up to 200 g / h. OJSC "Tambov Plant" Komsomolets "them. N. S. Artemova "Since 2005, it develops the production of carbon nanomaterial Taunit, which is a multi-line carbon nanotubes obtained by gas-phase chemical deposition on a metal catalyst. The total capacity of reactors for the production of carbon nanotubes of Russian manufacturers exceeds 10 t / g.
Nanopowders of metals and their connectionsare the most common type of nanomaterials, while their production is growing every year. In general, the methods of obtaining nanopowders can be divided into chemical(Plasmochemical synthesis, laser synthesis, thermal synthesis, self-propagating high-temperature synthesis (SVS), mechanochemical synthesis, electrochemical synthesis, precipitation from aqueous solutions, cryochemical synthesis) and physical (evaporation and condensation in an inert or reaction gas, electric explosion explosion (eVP), mechanical grinding, detonation processing). The most promising of them for industrial production are gas-phase synthesis, plasma chemical synthesis, grinding and electric explosion explosion.
For gas phase synthesis The evaporation of solid material (metal, alloy, semiconductor) was carried out at a controlled temperature in the atmosphere of various gases (AR, XE, N 2, not 2, air), followed by intensive cooling of the vary of the resulting substance. At the same time, a polydisperse powder is formed (particle size of 10-500 nm).
The evaporation of the metal can occur from the crucible, or the metal enters the zone of heating and evaporation in the form of wire, metal powder, or in the fluid jet. Sometimes the metal is sprayed with a bunch of argon ions. The supply of energy can be carried out by direct heating, transmitting an electric current through a wire, an electric arc discharge in plasma, induction heating currents of high and medium frequency, laser radiation, electron beam heating. Evaporation and condensation can occur in vacuo, in a fixed inert gas, in the gas stream, including in the plasma jet.
Thanks to this technology, performance reaches tens of kilograms per hour. In this way, metal oxides are obtained (MgO, A1 2 0 3, SIO), some metals (Ni, Al, T1, MO) and semiconductor materials with unique properties . The advantages of the method include low energy consumption, continuity, single-diet and high performance. The purity of nanopowders depends only on the purity of the feedstock. Traditionally, gas-phase synthesis is carried out in a closed volume at high temperature, therefore the risk of nanoparticles into the working area can be due only to an emergency situation or non-cellionism of operators.
Plasmochemical synthesis It is used to obtain nanopowders of nitrides, carbides, metal oxides, multicomponent mixtures with a particle size of 10-200 nm. In synthesis, the low-temperature (10 5 K) argon, hydrocarbon, ammonium or nitric plasma of various types of discharges (arc, glory, high-frequency and ultrahof-frequency) is used. In such a plasma, all substances decompose to atoms, with further rapid cooling of them, simple and complex substances are formed, composition, structure, and the state of which strongly depends on the cooling rate.
The advantages of the method are high speeds of education and condensation of compounds and great performance. The main disadvantages of plasma chemical synthesis are a wide distribution of particles in size (from tens to thousands of nanometers) and a high content of impurities in powder. The specifics of this method requires processes in a closed volume, therefore, after cooling, the nanopowdroke can enter the atmosphere of the working area only with incorrect unpacking and transportation.
Today, the semi-industrial level is implemented only physical Methods of obtaining nanopowders. This technologies own a very small part of the manufacturers, located, mainly in the United States, Great Britain, Germany, Russia, and Ukraine. Physical methods of obtaining nanopowders are based on the evaporation of metals, alloys or oxides with their subsequent condensation at controlled temperature and atmosphere. Parase transitions "Para-liquid-solid body" or "steam-solid" occur in the volume of the reactor or on a cooled substrate or walls. The starting material evaporates through intense heating, steam with the carrier gas is supplied to the reaction space, which is subjected to rapid cooling. Heating is carried out by plasma, laser radiation, electrical arc, resistance furnaces, induction currents, etc. Depending on the type of starting materials and the resulting product, evaporation and condensation are carried out in vacuo, in the stream of inert gas or plasma. The size and shape of the particles depends on the temperature of the process, the composition of the atmosphere and pressure in the reaction space. For example, in the atmosphere of helium particles have a smaller size than in an atmosphere of heavier gas - argon. The method allows you to obtain powders Ni, Mo, Fe, Ti, A1 with a particle size of less than 100 nm. Advantages, disadvantages and dangers associated with the implementation of such methods will be discussed below on the example of the wire explosion method.
The method is also widespread. grinding materials mechanically In which the ball, planetary, centrifugal, vibration mills, as well as gyroscopic devices, attributes and symoloomers are used. Technique and Disintegration Technology LLC produces fine powders, as well as nanopowders using industrial planetary mills. This technology allows you to achieve performance from 10 kg / h to 1 t / h, is characterized by low cost and high purity of the product controlled by the properties of particles.
Mechanically crossed metals, ceramics, polymers, oxides, fragile materials, and the degree of grinding depends on the type of material. So, for tungsten oxides and molybdenum, the particle size is about 5 nm, for iron - 10-20 nm. The advantage of this method is to obtain nanopowers of alloyed alloys, intermetallic, silicides and dispersed-strengthened composites (particle size of ~ 5-15 nm).
The method is easy to implement, allows to obtain material in large quantities. It is also conveniently that for mechanical methods of grinding, relatively simple installation and technologies are suitable, you can grind various materials and get alloys powders. The disadvantages include a wide distribution of particles in size, as well as contamination of the product with materials of the abrasive parts of the mechanisms.
Among all the listed methods, the use of choppers involves draining nanomaterials into the sewer after cleaning the devices used, and in the case of manual cleaning of parts of this equipment, the staff is in direct contact with nanoparticles.
- Laser ablation is a method for removing a substance with a surface pulse.
- Atrisors and simoloomers are high-energy grinding devices with a fixed body (drum with stirrers that make movement of balls in it). Atritamimizes vertical location of the drum, simoloomers -Gorizonal. Grinding grinding material with grinding balls, in contrast to other types of grinding devices, occurs mainly not for the runtime, but according to the mechanism of abrasion.
Restrictions on the use of nanomaterials
It turned out that materials with nanoscale grain differ in fragility. An important limitation for the use of nanostructured structural materials is their tendency to intercrystalline corrosion due to a very large volume fraction of grain boundaries. In this regard, they cannot be recommended for work in conditions of contributing to such corrosion. Another important limitation is the instability of the structure of nanomaterials, and, consequently, the instability of their physicochemical and physical and mechanical properties. So with thermal, radiation, deformation, etc. The impacts are inevitable relaxation, segregation and homogenization processes. When molding products from nanopowders, the problem of compete (sticking of nanoparticles) in agglomerates is also sustained, which may complicate the preparation of materials with a given structure and distribution of components.
It should be noted that the commercial market is currently
the most widely represented such nanomaterials like nanopowders
metals and alloys, nanopowders oxides (silicon, iron, antimony, aluminum, titanium), nanopowders of a row of carbides, carbon nanofibers, fullerene materials.
Nanodisperse objects are obtained in the form of sol, gel, concentrated dispersion or powder, thin film, nanoporous body. The range of methods for their preparation is extremely wide. Existing methods for obtaining nanobjects are classified according to the following features:
Synthesis strategy: Receipt may be based either on the dispersion process either on the condensation process - in foreign literature these methods are divided into two groups: "Top-Down" - "top down", i.e. Reducing the size, grinding, and "Bottom-UR" - "bottom up", i.e. the creation of nanostructures from smaller source components, more precisely from atoms and molecules (vividly both approaches illustrates Fig. 2.2);
The nature of the synthesis process (physical, chemical or biological);
Used in the synthesis of energy sources (laser, plasma, heating, freezing, mechanical, hydrothermal, combustion, etc.);
The medium in which nanoparticles or nanocrystals (NK) are formed (gas, liquid or sidewar).
The choice of this or that technology is determined by a number of factors, which includes the physical and chemical properties of the obtained particles, productivity, energy intensity of the process, environmental friendliness, etc.
The main methods for obtaining nanomaterials can be divided into a number of technological groups (Fig. 2.3): Powder-based methods
metallurgy, methods based on the production of amorphous precursors, surface technologies (the creation of coatings and modified layers with nanostructure), methods based on the use of intensive plastic deformation, and complex methods that use several different technologies sequentially or parallel.