Arsenic element refers to what elements. What is arsenic? Characteristic, properties and application
Finding in the nature of arsenic
Arsenic - scattered element. Content B. earth Kore 1.7 10-4% by weight. This substance may occur in native state, has the form of metallic brilliant gray shells or dense masses consisting of small grains. There are about 200 arsenic-containing minerals. In small concentrations, it is often contained in lead, copper and silver ores. Quite often there are two natural arsenic compounds with sulfur: orange-red transparent real kart ASS and lemon-yellow Auripigmentation AS2S3. Mineral having industrial importance - arsenopyrite (arsenic pechestan) FEASS or FES2 Feas2, also mined arsenic cchedan - Lullingite (Feas2).Get arsenic
There are many ways to obtain arsenic: sublimation of natural arsenic, the method of thermal decomposition of arsenic cchedan, the restoration of arsenic anhydride, etc. Currently, for obtaining metallic arsenic, the arsenopyrite in muffle furnaces is most often heated. At the same time, arsenic is exempt, the pairs of which are condensed and turn into a solid arsenic in iron tubes, which come from furnaces, and in special ceramics receivers. The residue in the furnaces is then heated when air access, and then arsenic turns into AS2O3. Metal arsenic is obtained in rather minor quantities, and main part Arsenic-containing ores are processed into white arsenic, that is, arsenic trioxide is arsenic anhydride AS2O3.Arsenic application
- The use of arsenic in metallurgy - is used to dop the lead alloys going to prepare the fraction, since when casting a fraction with a tower method, a mysterious alloy with lead acquire a strictly spherical shape, and in addition, the strength and solidity of the lead increase.
- Application in electrical engineering - arsenic of particular purity (99.9999%) is used to synthesize a number of practically very valuable and important semiconductor materials - arsenides and complex diamond-like semiconductors.
- Application as a dye - arsenic sulphide compounds - aurapygment and realgar - used in painting in quality paints.
- Application in the leather industry - used as a means to remove hair with leather.
- Application in pyrotechnics - realgar is used to obtain "Greek", or "Indian", fire arising from burning a mixture of Realgar with gray and Selitra (bright white flame).
- Application in medicine - Many of the arsenic compounds in very small doses are used as drugs to combat anemia and a number of serious diseases, as they have a clinically significant stimulating effect on a number of functions of the body, in particular, to bleeding. From ne. organic compounds Arsenic arsenic anhydride can be used in medicine to prepare a pill and in dental practice in the form of pasta as a necrotic drug (the very "arsenic", which is laid in the tooth channel before the nerve removal and sealing). Currently, arsenic drugs are used in dental practice rarely due to toxicity and the possibility of making painless tooth denervation under local anesthesia.
- Application in glass production - arsenic trioxide makes the glass "deaf", i.e. opaque. However, small additives of this substance, on the contrary, brighten the glass. Arsenic and now enters the recipes of some glasses, for example, the "Vienna" glass for thermometers and twist.
The most promising area of \u200b\u200bthe use of arsenic, undoubtedly semiconductor technique. Of particular importance was the ARSENIDES GALLY GAAS and India Inas. Arsenide Gallium is also needed for an important direction of electronic equipment - optoelectronics, which arose in 1963 ... 1965. At the junction of physics solid, optics and electronics. The same material helped create the first semiconductor lasers.
Why did the arsenids prosesented for semiconductor equipment? To answer this question, we remind briefly about some of the basic concepts of semiconductor physics: "valence zone", "prohibited zone" and "conduction zone".
Unlike a free electron, which can possess any energy, an electron, enclosed in an atom, can only have some, well-defined energy values. Of the possible values \u200b\u200bof electron energy in the atom there are energy zones. By virtue of the well-known principle of Pauli, the number of electrons in each zone cannot be more than a certain maximum. If the zone is empty, then it naturally cannot participate in the creation of conductivity. Do not participate in conduction and electrons entirely filled zone: since there are no free levels, the external electric field cannot cause the redistribution of electrons and thereby create electricity. Conductivity is possible only in a partially filled zone. Therefore, the bodies with a partially filled zone belong to metals, and bodies that energy spectrum Electronic states consists of filled and empty zones - to dielectrics or semiconductors.
Recall also that the entire zones in crystals are called valence zones, partially filled with and empty - conduction zones, and the energy interval (or barrier) between them is a prohibited zone.
The main difference between dielectrics and semiconductors is precisely in the width of the prohibited zone: if it needs more than 3 eV to overcome it, then crystal refers to dielectrics, and if less - to semiconductors.
Compared with the classic semiconductors of the IV group - Germany and silicon - the arsenides of the elements Group III Possess two advantages. The width of the forbidden zone and the mobility of charge carriers in them can be varied in broader limits. And the more movable charge carriers, the semiconductor device can work at high frequencies. The width of the forbidden zone is chosen depending on the appointment of the device.
Thus, for rectifiers and amplifiers, designed to work at elevated temperatures, a material with a large width of the forbidden zone is used, and for cooled receivers infrared radiation - With small.
Gluff Arsenide acquired a special popularity because it has good electrical characteristics that it retains in a wide temperature range from minus to plus 500 ° C. For comparison, we indicate that India's arsenide, not inferior to GaAs by electrical properties, starts to lose them at room temperature, Germany - at 70 ... 80, and silicon - at 150 ... 200 ° C.
Arsenic is used as a doping additive, which gives "classic" semiconductors (Si, GE) a conductivity of a certain type. In this case, the so-called transition layer is created in the semiconductor, and, depending on the purpose of the crystal, it is doped so as to obtain a transition layer at different depths. In crystals intended for the manufacture of diodes, its "hiding" deeper; If the solar panels will be made from semiconductor crystals, the depth of the transition layer is not more than one micrometer.
Arsenic as a valuable additive is used in non-ferrous metallurgy. Thus, the additive to the lead 0.2 ... 1% AS significantly increases its hardness. The fraction, for example, is always done from lead, doped with arsenic - otherwise not to get a strictly spherical shape of crushers.
Supplement 0.15 ... 0.45% of arsenic in copper increases its tensile strength, hardness and corrosion resistance when working in a rolled medium. In addition, arsenic increases copper fluidity during casting, facilitates the process of wire dragging.
Add arsenic into some varieties of bronze, brass, babbitis, typographic alloys.
And at the same time, arsenic very often harms metallurgists. In the production of steel and many non-ferrous metals, intentionally go to the complication of the process - just to remove all arsenic from metal. The presence of arsenic in ore makes the production of harmful. Harmful twice: first, for people's health; Secondly, for metal - significant impurities of arsenic worsen the properties of almost all metals and alloys.
All compound. arsenic, r-role in water and weakness media (eg, gastric juice), extremely poisonous; MPC in the air arsenic and its comprehensive. (except ash3) in terms of arsenic 0.5 mg / m3. Seda. AS (III) more poisonous than compound. AS (V). From noorg. Seda. AS2O3 and ASH3 are especially dangerous. When working with arsenic and its social. Needed: full sealing of equipment, removal of dust and gases intense ventilation, compliance with personal hygiene (anti-high clothing, glasses, gloves, gas masks), frequent medical control; Women and adolescents are not allowed to work. In acute poisoning, arsenic observed vomiting, abdominal pain, diarrhea, oppression center. nervous system. Help and antidote in arsenic poisoning: Reception of aquatic p-mms Na2S2O3, washing the stomach, reception of milk and cottage cheese; Specific. Antidote - Unitiol. A special problem is to remove arsenic from outgoing gases, tehnol. Waters and side products of processing ores and concentrates of non-ferrous and rare metals and iron. Naib The method of burial arsenic is promising by translating it into almost insoluble sulfide glass.
Arsenic is famous with deep antiquity. An Aristotle mentioned his priest. Survous connections. It is not known who first received elementary arsenic, usually this achievement is attributed to Albert Great Ok. 1250. Chem. The element of arsenic is recognized by A. Lavoisier in 1789.
Such is the element No. 33 deservedly enjoyed by a bad reputation, and yet in many cases very useful.
The content of arsenic in the earth's crust is only 0.0005%, but this element is quite active, and therefore minerals, which includes arsenic, over 120. The main industrial mineral of arsenic - Arsenopyritis Feass. Large copper-arsenic fields are in the USA, Sweden, Norway and Japan, arsenic-cobalt - in Canada, arsenic-tin - in Bolivia and England. In addition, golden-ryyaky deposits in the USA and France are known. Russia has numerous mysteria deposits in Yakutia and the Caucasus, in Central Asia And in the Urals, in Siberia and in Chukotka, in Kazakhstan and in Transbaikalia. Arsenic is one of the few items, the demand for which is less than the possibility of their production. World arsenic (without socialist. Countries) in terms of AS2O3 approx. 50 thousand tons (1983); Of these, ~ 11 tons of elementary arsenic of particular purity for the synthesis of semiconductor compounds are obtained.
X-ray fluorescent arsenic analysis method is quite simple and safe, unlike chemical method. A pure ball is pressed into the tablet and is used as a standard. GOST 1293.4-83, GOST 1367.1-83, GOST 1429.10-77, GOST 2082.5-81, GOST 2604.11-85, GOST 6689.13-92, GOST 11739.14-99 Definition is performed using a X-ray fluorescent spectrometer. The most proven in this area is the EDX 3600 B and EDX 600 spectrometers.
Arsenic (the name happened from the word mouse, was used to persuade mice) - thirty-third element of the periodic system. Refers to semimetillands. In a compound with acid, it does not forms salts, being an acid-forming substance. May form allotropic modifications. Arsenic has three famous crystal lattice structures today. Yellow arsenic shows the properties of a typical nonmetalla, amorphous - black and the most steady metallic, gray. In nature, most often occurs in the form of connections, less often - in a free state. The most common are arsenic compounds with metals (arsenides), such as: arsenic iron (arsenopyritis, poisonous pechestan), Nickelin (Kurfernikel, is named so because of its similarity with copper ore). Arsenic is an inactive element, insoluble in water, and its compounds relate to undermining substances. The oxidation of arsenic occurs during heating, at room temperature this reaction proceeds very slowly.
All arsenic compounds are very strong toxins that have a negative impact not only at the gastrointestinal tract, but also on the nervous system. Stories are known many sensational cases of poisoning arsenic and its derivatives. The arsenic compounds were used as a poison not only in medieval France, they were known yet in ancient Rome, Greece. The popularity of arsenic as a potent poison is explained by the fact that it is almost unrealistic to detect it in food, it does not have a smell or taste. When heated, turns into arsenic oxide. To diagnose arsenic poisoning is rather difficult, as it has similar symptoms with various diseases. Most often, arsenic poisoning is confused with cholera.
Where is arsenic applied?
Despite its toxicity, arsenic derivatives are used not only to escape mice and rats. Since pure arsenic has high electrical conductivity, it is used as a doping additive, which gives such semiconductors as Germany, silicon conductivity of the required type. In non-ferrous metallurgy, arsenic is used as a additive that gives alloys strength, hardness and corrosion resistance in the rolled medium. It adds it in small quantities to clarify the glass, in addition, it is part of the famous "Vienna Glass". Nickelin is used for glass color in green. In the leather business, sulphate arsenic connections are used when processing the skins for removing hairs. Arsenic is part of varnishes and paints. In the woodworking industry use arsenic as an antiseptic. In pyrotechnics from the sulfide compounds of arsenic make "Greek fire" are used in the production of matches. Some arsenic compounds are used as combat poisoning substances. Toxic properties Arsenic is used in dental practice to kill the tooth pulp. In medicine, arsenic preparations are used as a medicine that increases the overall tone of the body, to stimulate an increase in the number of red blood cells. Arsenic has an inhibitory effect on the formation of leukocytes, so it is used in the treatment of some forms of leukemia. A huge amount of medical preparations created on the basis of arsenic is known, but recently less toxic drugs are gradually replaced.
Despite its toxicity, arsenic is one of the most elements necessary. When working with its compounds, it is necessary to adhere to the safety regulations, which will help to avoid unwanted consequences.
Arsenikum or Arsenik - such a name on Latin has arsenic in chemical tables. In Russian, the word arsenic appeared after the oxide of this substance was used in the struggle against mice and rats. Arsenic has the form of very small shells with a metal glitter or dense formation of small grains. One of his inorganic compounds is arsenic anhydride - is widely used in medical, in particular dental practice.
How and for what a dentist uses arsenic
This substance is used by doctors to obtain an anesthetic effect. The drug with arsenic kills the nerve of a patient tooth, of course there are other means to obtain the same effect, but this method is still continuing to use because it is effective and tested by decades.
Under the enamel of the tooth enamel and dentin (solid tooth fabric), which constitutes its foundation, is a pulp. It consists of a variety of nerve endings and blood vessels. With acute pulpit, inflammation and swelling occurs, which squeezes nerve endings, hence severe pain.
On a note!Dental enamel is the durable biological tissue, boring drills therefore made using diamond.
Arsenic provides:
- necrotic effect on all nerve endings in the tooth;
- pulp overalls;
- cessation of blood supply;
- termination of pulses from nerve endings.
The arsenic paste contains anesthetic, so the process of exposure to arsenic flows painlessly.

The composition of the paste may vary depending on the manufacturer. The approximate composition of the drug is:
- arsenic anhydride;
- novocaine, Lidocaine or other anesthetic;
- antiseptic type camphor;
- tanin, viscous substance extending arsenic effect.
If severe pain worries, then a anesthetic agent can additionally overlap on top of the paste.
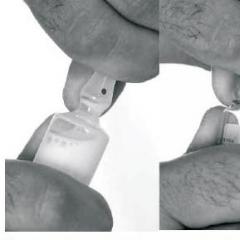
The doctor drills the tooth, cleans it and makes a drug into the cavity. Then closes the temporary seal, with which the patient walks depending on the instructions of the doctor. This can be from 1 to 5 days.
On a note!The ingress of arsenic from the cavity of the tooth into the oral cavity should be excluded, as this can lead to osteomyelitis.

During the aims of the arsenic nerves inside the tooth can affect the occurrence of the new pain, it can last for several hours, a bromide preparation is taken for anesthesia. After the prescribed time, the doctor will reach a temporary seal, remove the arsenic, destroyed nerve and captures the prepared cavity of the tooth.
Influence of arsenic
In the tissues where arsenic anhydride is valid, a violation of the normal breathing of cells can occur. Even a small amount of drug affects the extension of vessels and can lead to hemorrhages. In the nerve fibers, the majority of components occur. Such changes are directly proportional to the dosage of the substance and the term of its impact. The preparation with arsenic is used when there is a need to remove nerves and pulp.
On a note!Alcohol's use is absolutely prohibited after the laying of arsenic paste, as its impact is enhanced and the risk of intoxication becomes very likely.

Indications and contraindications
The substance is widely used by state clinics as an efficient and most affordable tooth-nerve agent. Also, the drug is used when:
- the impossibility of performing another kind of anesthesia;
- the need to emerge nerve;
- allergies to other painkillers;
- ineffectiveness of other painkillers;
- the presence of individual indications;
- in children's dentistry only with the roots formed.
Arsenic paste is not used in case:
- childhood up to one and a half years;
- allergic reaction to the drug;
- pregnancy;
- diseases of urinary organs;
- threats of glaucoma;
- breastfeeding;
- lack of full cleaning channels;
- curvature of the dental channel;
- disorders of the integrity of the roots of the teeth.

On a note!Traces of some metals in the body, including arsenic can play a role in the pathogenesis of glaucoma.
If a tooth with arsenia
If the dental pain continues more than a day, then you should immediately contact the dentist. Such a reaction may occur in the following cases:
- allergies on arsenic or other components;
- the doctor put arsenic on the closed pulp;
- inflammation or necrosis of tissue around the tooth;
- low concentration of substance;
- presence of periodontitis;
- violations in the technology of applying substances;
- high sensitivity in which pain can serve in a few days.
With severe pain, especially at night, it is better to seek help. With inflammation of the tissues around the tooth or necrosis caused by arsenic, very dangerous states affecting the periosteum or bones of the jaw may occur.
On a note!On the first day after laying arsenic in pain, you can drink a tablet of any painkillers.
If arsenic fell
There are situations where during the meal time seal is destroyed and arsenic falls out. Immediately after that, you need to rinse the oral cavity with a soda solution with the addition of iodine, this is done to neutralize the possible residues of anesthetic paste. Then the tooth cavity must be closed with a cotton ball and contact the dentist.
In other situations, arsenic may be accidentally swallowed, but the dosage of the drug is such that it will not cause negative consequences in the form of intoxication. In order not to worry about this, you can drink milk, or take activated coal. The seal with arsenic can fall out when not compared with the doctor's recommendations, they include:
- Within two hours after visiting the doctor, do not eat.
- If an acidic taste appears on a seal, rinse the soda solution.
- Try not to chew on the side of the sick tooth or take up soft food.
- Be sure to visit the doctor at a specified period for the removal of arsenic, temporary seal and continuing treatment.
On a note!If you exceed the time of finding arsenic in the cavity of the tooth it is possible to develop necrosis of tissues around the tooth in patients with diseases of the digestive and increased sensitivity to the drug possible the development of intoxication.
Video - a specialist about arsenic in the tooth
Independent getting rid of arsenic
It is possible to get rid of the paste, but undesirable. This should be done only in extreme cases when assistance is required, but for some reason it is impossible to receive it in a timely manner.
If you want to remove the temporary seal, it can be done using a needle from a syringe or any other. Arsenic is removed with its help, the needle must be treated with alcohol. The oral cavity after that is rolling several times a day with a solution of soda with several drops of iodine. Open tooth must be covered with a piece of watts and contact the dentist as soon as possible.
The consequences of exceeding the dose of arsenic
If the dose was exceeded by a doctor or the patient revealed and did not appear on time to remove arsenic, then possibly negative consequences, the most common of them:
- pulp swelling;
- darkening of solid tooth tissue;
- periodontitis;
- osteonecrosis;
- general intoxication.
Given all the consequences, arsenic-based drugs are not applied to pregnant and lactating women, arsenic is also practically not used to treat children's teeth.
On a note!In the case of the treatment of children, it is difficult to calculate the necessary dose of arsenic paste, also a child can independently navigate the seal and swallow arsenic.
Comparison of arsenic and clarificate pastes
Paste with arsenic Features 30% The content of arsenic anhydride. Used in the propagation of carious process through a thin tooth tissue, when infected with the pulp. Maximum period of leaving pastes in the tooth 3 days The maximum period of leaving paste in the teeth of 7 days. It consists in addition to the active substance from lidocaine, camphor, ephedrine, chlorophenol. Athletes are not recommended to use, can show a positive reaction to anti-doping control. Formaldehyde-based paste Such pastes, in contrast to the arsenic, can mummify the pulp, but are still considered less effective
As part of paraformaldehyde, lidocaine, creosote. Time actions from 2 to 7 days Contains paraforms, chlorophenol, menthol, camphor, lidocaine is used on milk teeth, allows not to remove the pulp As part of lidocaine, paraformaldehyde, phenol. Applied from 7 to 10 days In the dental clinic, the doctor will use an anesthetic for individual indications and will not put arsenic without your consent.
Arsenic
ARSENIC -but; m.
1. Chemical element (AS) - solid poisonous substance brilliantly gray, which is part of many minerals. Oxidized arsenic. Get arsenic.
2. A drug containing this substance or its compounds (applied as a constructive, antimicrobial, etc. means). Arsenic treatment. The impact of arsenic for nervous endings.
◁ Arsenic ,y ,y. Mth compounds. Mr. Acid. M. Preparation. M-oxyment. Arsenic ,y ,y. Study. Arsenic, and ,y. ● The Russian name of this element occurred from the word "mouse", because The arsenic was widely used in the destruction of rats and mice.
arsenic(Lat. ARSENICUM), chemical element V group of periodic system. Russian name from "Mouse" (arsenic preparations were used to exterminate mice and rats). Forms several modifications. Ordinary arsenic (so-called metallic, or gray) - fragile crystals with silver glitter; The density is 5.74 g / cm 3, at 615 ° C is removed. The air is oxidized and fastens. Mining from sulphide ores (Arsenopyritis minerals, auripigment, realgar). Component of alloys with copper, lead, tin, etc. and semiconductor materials. Arsenic compounds are physiologically active and poisonous; served as one of the first insecticides (see, for example, the arsenates of metals). Inorganic compounds Arsenic applied in medicine as constructing, tonic products, organic - as antimicrobial and anti-polocular (in the treatment of syphilis, amoebiasis, etc.).
ARSENICArsenic (lat. Arsenicum, from Greek ARSEN - strong), as (read "Arsenikum"), chemical element with atomic number 33, atomic mass 74,9216. In nature, one stable isotope 75 AS is found. Located in VA group in 4 periodic system of elements. Electronic Configuration External Layer 4 s. 2
p. 3
. The degree of oxidation +3, +5, -3 (valence III, V).
Radius of an atom 0.148 nm. Ion radius AS 3-0.191 nm, ion AS 3+ 0.072 nm (coordination number 4), AS 5+ ion 0.047 nm (6). Energy of successive ionization 9.82, 18.62, 28.35, 50.1 and 62.6 eV. Electricity by Pauling (cm. Paulong Linaus) 2.1. Non-metal.
Historical reference
Arsenic is known for mankind from ancient times when the Minerals are used as dyes Auripigmentation (cm. Auripigmentation) AS 2 S 3 and Realgar (cm. REALGAR)AS 4 S 4 (mentions are found at Aristotle) (cm. ARISTOTLE).
Alchemists when calculating the sulphides of arsenic in air, noted that the formation of the so-called white oxide AS 2 O 3:
2as 2 S 3 + 9O 2 \u003d 2As2O 3 + 6SO 2
This oxide is a strong poison, it dissolves in water and in wine.
For the first time as in free form received German alchemist A. Bldstdt in the 13th century Warming arsenic oxide with coal:
AS 2 O 3 + 3C \u003d 2AS + 3SO
For the image of arsenic used a sign of a silent snake with a disclosed mouth.
Finding in nature
Arsenic - scattered element. Contents in the earth's crust 1.7 · 10 -4% by weight. 160 arsenic-containing minerals are known. In native state, it is rare. Mineral having industrial importance - arsenopyrite (cm. Arsenopyritis) Feas. AS is often found in lead, copper and silver ores.
Obtaining
The enriched ore is subjected to oxidative firing, then sublimated the volatile AS 2 O 3.. This oxide is reduced by carbon. To clean the AS, it is distilled off in vacuo, then translated into ASCL 3 volatile chloride, which is reduced by hydrogen (cm. HYDROGEN). The resulting arsenic contains 10 -5 -10 -6% impurities by weight.
Physical and chemical properties
Arsenic - gray with a metal brilliance fragile substance (A-arsenic) with a rhombohedral crystal lattice, a. \u003d 0.4135 nm and a \u003d 54,13 °. Density 5.74 kg / dm 3.
When heated to 600 ° C AS sublimates. When cooling vapors, a new modification occurs - yellow arsenic. Above 270 ° C, all forms AS are moving into black arsenic.
You can melt as possible only in sealed ampoules under pressure. The melting point is 817 ° C with a pressure of its saturated vapor of 3,6 MP.
The structure of gray arsenic is similar to the structure of the gray antimony and the structure resembles a black phosphorus.
Arsenic is chemically active. When stored on air, the powdered AS flammives with the formation of AS 2 O 3 acid oxide. This oxide in pairs exists in the form of dimers AS 4 O 6.
With careful dehydration of arsenic acid H 3 ASO 4, the highest acidic oxide of arsenic AS 2 O 5, which, when heated, easily gives oxygen (cm. OXYGEN), Turning into AS 2 O 3.
AS 2 O 3 oxide corresponds to existing ocomyshydic H 3 ASO 3 solutions and federic weak acids Haso 2. Their salts are arsenates.
Diluted nitric acid (cm. NITRIC ACID) Oxidizes AS to H 3 ASO 3, concentrated nitrogen acid - to H 3 ASO 4. Alkali AS does not react, dissolves in water.
When heated AS and H 2, gas Arsin is formed (cm. Arsenic hydride) ASH 3. With fluorine (cm. FLUORINE) and chlorom (cm. CHLORINE) AS interacts with self-ignition. When interacting as with gray (cm. SULFUR)selenium (cm. SELENIUM) and Telllur (cm. TELLURIUM) CHALGOGENIDES FORMAGE: (cm. Chalcogenides) AS 2 S 5, AS 2 S 3, AS 4 S 4, AS 2 SE 3, AS 2 TE 3, existing in a glassy state. They are semiconductors.
With many Metals AS forms arsenide (cm. ARSENIDES). Arsenide Gaas Gaas and India Inas - semiconductors (cm. Semiconductors).
It is known a large number of organic compounds of arsenic in which there is chemical communications AS - C: OrganoCins R N. Ash. 3-N. (n.\u003d 1,3), Tetraorganodiecins R 2 AS - ASR 2 and others.
Application
AS special purity is used to synthesize semiconductor materials. Sometimes AS is added to steels as a doping additive.
In 1909 German microbiologist P. Erlich (cm. Erlich Paul) Received "Preparation 606", effective medicine from malaria, syphilis, returning title.
Physiological action
Arsenic and all its connections to poisonous. In acute poisoning, arsenic observed vomiting, abdominal pain, diarrhea, inhibition of the central nervous system. Help and antidote with arsenic poisoning: Reception aqueous solutions Na 2 S 2 O 3. Washing the stomach, the reception of milk and cottage cheese; Specific antidote - unitiol. MPC in the air for arsenic 0,5mg / m 3. Work with arsenic in hermetic boxes using protective overalls. Due to the high toxicity of the arsenic connection, Germany was used as poisoning substances in the First World War.
In the territories where in the soil and water excess arsenic, it accumulates in the thyroid gland in humans and causes endemic goiter.
encyclopedic Dictionary. 2009 .
Synonyms:Watch what is "arsenic" in other dictionaries:
ARSENIC - (ARSENUM, ARSENIUM, ARSENI CUM), solid metalloid, sim. AS; at. in. 74.96. In the periodic system of elements, it takes in order 33 E place, in 5 m row of V groups. Natural compounds M. with gray (Realgar and Auripigmentation) were still known in ... ... Big medical encyclopedia
ARSENIC - See arsenic (AS). As arsenic and its compounds are widely used in the national economy, it is contained in the wastewater of various industries of the metallurgical, chemical pharmaceutical, textile, glass, leather, chemical ... Fish diseases: Directory
Arsenic - (crude arsenic) is a solid extracted from natural arsenopyrites. It exists in two main forms: a) ordinary, so-called metallic arsenic, in the form of brilliant steel crystals, fragile, not ... Official terminology
- (symbol AS), poisonous semimetallic element of the fifth group of the periodic table; It was probably obtained in 1250 compounds containing arsenic, used as an invocoule for rodents, insects and as a means against weeds. They also apply ... Scientific and technical encyclopedic Dictionary
- (ARSENIUM), AS, chemical element V group of periodic system, atomic number 33, atomic weight 74,9216; Nonmetall gray, yellow or black, TPL 817 shc, is removed at 615 shc. Arsenic is used to obtain semiconductor ... ... Modern encyclopedia
Arsenic - (ARSENIUM), AS, chemical element V group of periodic system, atomic number 33, atomic weight 74,9216; Non-metal gray, yellow or black, TPL 817 ° C, is removed at 615 ° C. Arsenic is used to obtain semiconductor ... ... Illustrated Encyclopedic Dictionary
ARSENIC - Chem. Element, symbol AS (lat. Arsenicum), at. n. 33, at. m. 74.92; Nemmetal, exists in several allotropic modifications, density 5720 kg / m3. Under normal conditions, the most chemically racks are so-called metallic, or gray, arsenic. ... ... Large polytechnic encyclopedia
As - a sign of mankind from ancient times. Already the Great Aristotle mentioned the chemical element of arsenic in connections natural character. In addition, the possibility of developing its sulfur variety, by calcination, is described by DIOSCRIDE in the first century to our era.
Later, with this element, European steel mills encountered when working with ore, with mysterious enclosures. Alchemists studied him very closely. Such attention was explained by the fact that he also as sulfur and mercury referred to the natural elements that are the basis of all metals.
Arsenic's ability to change color from copper alloys on white was perceived by professors of modern chemistry as copper metamorphosis in silver. Now in the world no laboratory is without this element.
AS is present everywhere. Even smoking cigarette has its maintenance that, among other things, causes the harmfulness of smoking.
History opening
The opening of a muscular based on a metal basis refer to the eighteenth century, however, the method of obtaining an element with sublimation will be revealed only by the end of the seventeenth. During this period, Chemik Shelele discovered arsenic acid, as well as hydrogen present in it.
The study of organic compounds with the content of AS originates from a chemist. In the middle of the eighteenth century, he receives the first combination of an organic nature based on it - "Liquid Cadi". The structure was dismantled to the constituents only after eighty years, another famous chemist Bunzen.
There are still disputes about who to give the palm to the opening of the element in its pure form. This achievement is counted in the merit of Albert Great. As a chemical element, he was recognized by Lavoisier in 1789.
Production and applications

Modern specialists know about two hundred minerals in which there is arsenic. In the overwhelming majority of cases, it is found in ore containing copper, silver or lead. However, the mineral representing the main importance for the industry is the cchedan with the presence of arsenic.
There are several ways to generate AS on an industrial scale. The main type of production was the court of arsenopyrite. Next, the oxide is restored from anthracite.
However, most of the raw materials are transformed into arsenic white.
Arsenic in the dental sphere

This chemical element is not only poison, but also the medicine.
The use of arsenic in the field of dentistry in the form of a paste has not lost its significance due to the bright necrotic effects of the substance on the affected fabrics.
Apply it in the following cases:
- If the patient does not perceive anesthetics;
- In cases of contraindications of anesthetic drugs;
- When curing the dental pain in children.
The main condition when using it in dental clinics is a fully formed root system. Therefore, the "children's" application option is not so common.
AS in industry

The chemical element of arsenic is used in many areas of production, among which several main directions can be distinguished:
- Metallurgy;
- Electrical engineering;
- Skin treatment;
- Textile industry;
- Pyrotechnics;
- Glass production.
Metallurgy - Apply for doping lead alloys used for the manufacture of fractions. Such an alloy with the addition of AS with the throat version of the production allows you to receive ideal spherical shapes of crushing. In addition, its strength increases.
Electrical engineering - High purification arsenic (up to 99%) is used to manufacture a number of necessary semiconductor components.
Textile industry - Used as a dye.
Tanne industry - In this area, it is used as a reagent for the destruction of bristles on the skin.
Pyrotechnics - Realgar mineral, which is a muscular monosulfide, is used for the manufacture of "Greek" fire, resulting from a mixture of it with gray and Selitra. That chemical compound Gives a bright white flame.
Glass case - Thunderies AS allows you to get products having zero transparency. Meanwhile, the small addition of the component, on the contrary, brighten it. This element has so far remained part of the production of some glasses.
For example:
- "Vienna";
- used in thermometers;
- Imitation of crystal.
In addition, arsenic is used in agriculture as fertilizer. The home method is a rat poison. Now it is made on the basis of other components.
Eating is strictly prohibited.
Arsenic in the fight against leukemia

Famous Employers Arsenic's ability to kill cells is now used for noble purposes. This chemical element is widely used to treat oncological diseases, first of all during leukemia.
Leukemia is characterized by the formation of a tumor due to the replication of the bone marrow. In the absence of timely treatment, it increases its volume. For this reason, metastases arise and grow in all parts of the body. Even the difficult form of the disease helps an AS element.
It effectively neutralizes excessive heating of leukocytes, stimulates the rapid and high-quality formation of red taurus. All this allows you to positively influence the recovery process. In the treatment of this dangerous element, clear instructions should be observed. After all, the final price is a person's life.
Possible causes of poisoning

Nowadays there is a big risk to poison arsenic. None of the workers in production are insured from different surprises. When using substances based on AS in domestic conditions, there is also a chance of random to get into the human body.
Sometimes the facts of deliberate poisoning are recorded - criminal offenses or suicide. These episodes can be attributed to the sharp forms of intoxication.
There is probability to poison and with therapeutic practice of exposure to small doses. Such poisoning is counted for chronic cases.
A separate group of intoxication by this chemical element is a prosthetic category. When a person is present in places where there is a large concentration of Adamcite.
It uses police in some countries when overclocking demonstrations. Combat poisoning substances are divided into several categories including - Sterny. Arsenic applies to them. Such substances act annoyingly to human breathing apparatus.
Alert arsenic

The element has the ability to quickly penetrate the human body, and it is very difficult to output it.
The poisoning is happening in the following ways:
- Skin coat;
- Lungs;
- Gastrointestinal tract.
It is worth noting that the inorganic components of arsenic are absorbed far faster than organications.
The greatest danger to a person represents an Arsin in a gaseous state, it does not smell, therefore, for its industrial production, it is necessary to make special additives having a resistant garlic "aroma". Also dangerous arsenic hydrogen.
Poisoning occurs very quickly. During the day, the element is able to hit internal organs. Two weeks after intoxication, the tracks of arsenic can be found in the nails and even in the bones.
Symptoms of arsenic poisoning

Signs of the disease can be varied depending on the adopted dose.
- Acute form
It is characterized by a metal resistant taste in the mouth. A person feels a strong throat burning, accompanied by spasms. The skin on the body acquires a shiny tint, and the palms are yellow.
Arterial pressure drops sharply, accompanied by powerful dizziness attacks. In addition, the poisoned is experiencing acute renal and liver failure.
Also, the patient has diarrhea and begins to hurt the stomach. Diarrhea is characterized by an acute form, as a result of which the body is very quickly dehydrated. In extreme cases, the probability of pulmonary edema, paralysis or comatose state is high.
- Prostrate form
There is an extremely acute headache. All mucous membranes, especially eye and respiratory tract, get strong irritation. This leads to a "cold", nasal congestion and tear.
The victim often sneezes and coughs. Strong nausea and even vomiting is also not excluded. After spasms in the mouth remains aftertaste with a metal tint.
- Chronic form
There is fatigue and the general ailment of the body. Limbs weaken on the background of anemian state. Peripheral sensitivity deteriorates until full loss. The skin is "running goosebumps" and its numbness is felt.
Sparies from vessels appear on the body and the development of stable cuperosis is being developed.
In the absence of appropriate treatment, serious consequences are very likely to toxic hepatitis. Since arsenic has a high carcinogenicity, the poisoning can push the development of oncology in the body.
For a person who swallowed arsenic triumside, the deadly dose will be a volume of 50 to 340 milligrams. It is tied to the type of substance and is directly related to the weight of man and the general state of health.
First aid for poisoning

If you or someone from loved ones or colleagues accidentally poisoned arsenic, immediate assistance should be provided before the arrival of specialists.
Actions are carried out according to a simple algorithm:
- The first thing to do is to immediately call the ambulance brigade;
- Before the arrival of doctors, the victim give a vomit to rinse the stomach;
- The next step will be the reception of the absorbent (for example, milk with whipped protein or activated carbon);
- On the stomach of the victim to put a hot height;
- If possible, prepare a special solution consisting of one spoon of magnesia with a burning, 200 ml of water;
- In no case, in no case, the victim can not give a sniffing alcohol or sour drinking;
- When convulsion appears, the victim's limb is the victim.
AS is a strong poison that can suffer great harm.
Unitol became the main antidote for arsenic. This effective antidote having a property to bind it into safe connections and allows you to get rid of chemical element with urea.
To remove the toxicological effect of arsenic when work in production helps and timely prevention measures.
How to warn poisoning

In the prevention of poisoning, try to avoid products with its content. In the workplace, industrial processes are sealing and ventilation improvement.
Personal hygiene plays a huge role in the prevention of poisoning. In the workplace it is necessary to use the respirator. Or apply tampons, from wool, which are laid in ears and nostrils. After work, it is necessary to wash. In addition, watch for your overalls. Keep it clean and washed.
Mandatory prophylactic measure should be a regular medical examination. Such surveys are recommended to be held at least twelve months with constant contact with the preparations containing arsenic.