Misunderstanding. Blue blood in a person, or who kicaneics are kinetics in psychology
The founder of the kinetics - science learning the language of gestures is Professor F. L. Birdwistell, engaged in permanent analysis of gestures and movements, came to the conclusion that the language of gestures, if he correctly understood the person to which they are addressed, can be expressed by the idea of \u200b\u200bnot worse than words. Research Birdwistell is supported by studying movements conducted by scientists different countries. Here are their conclusions:
If a person spends his hand on her lips, then he does not want to answer the question.
If he raised his nose, then it means that he decided.
If, when conversing a person folds his fingers in the form of the tower, it means that it is in indecision.
Consent: a person strokes herself through her hair.
If a person puts on the face of an impermeability mask - this means a complete denial.
A lady sitting with crossed legs, while rotating one leg - a sign of interest in a man present.
A man who listens to you, the legs are crossed at the same time, and he relies on his hand, is configured critically and skeptically.
If a person is difficult to observe silence, he crosses his hands in front of her lips.
If the speaker with both hands covers the tribune standing in front of him, he is aggressive, but not confident. If a person spreads his legs wide, and thumbs sticks in a hinge from a vest, he is very confident. He who keeps one hand with one hand, satisfied with himself. Orator who puts his fingers with force, wants to achieve an understanding from his listeners.
Demagogues extend the arms forward, as if someone had hypnotized them.
Hope is looking forward, fatigue and despair - down. Movements sent out, talk about honesty and kindness.
The opposite direction movements are inside - they talk about obstacles or that the personality of this person is distinguished by a closedness.
Handshake, causing pain - the expression of complete unceremonia and falsehood.
Strong handshake, but not excessive - honesty and reliability.
A weak powerless hand is a sign that this person can easily manage from the side. Fast charge when the hand of another person comes to your one moment, - indifference and closedness.
If a person turns a spoon in a cup with milk, tea or coffee to the left, that is, counterclockwise, it is extremely successful in life.
If a person after sat on the chair, immediately crossed his hands on his chest, then this is a sign of a closed character. Hand-crossed behind their backs indicate that a person retreated before circumstances surrendered. If a person wants to hide something (for example, at customs), he begins to whistle, or in the heat will put off the coat, or sunglasses, or will burn his jaw.
KINETICS
KINETICS
(Greek, from kinesis - movement). Science, which studies the dependence between the movement of matter and the reasons that cause these movements.
Vocabulary foreign wordsincluded in the Russian language. - Chudinov A.N., 1910 .
KINETICS
greek, from kinesis, movement. Motion science.
Explanation of 25,000 foreign words included in the Russian language, with their roots. - Michelson A.D., 1865 .
Kinetics
(c. Kinetikos leading in motion) The mechanics section in which the movement is studied depending on the physical. reasons for its determinants;
to. Physical - section of physics, in which the processes in bodies occur in the violation of thermal, mechanical, electrical, etc. of the equilibrium state;
k. Chemical - section of physical chemistry, in which the speed of Him is being studied. Reactions and those intermediate products that are formed during the reaction flow.
New dictionary foreign words.- by edwart,, 2009 .
Kinetics
kinetics, mn. No, g. [ from Greek. Kinetikos - Movement] (fur.). Department of mechanics, hugging dynamics and statics.
Big Dictionary Foreign Words. - PDDK Publishing House, 2007 .
Kinetics
Dictionary Foreign words L. P. Krycin.- M: Russian, 1998 .
Synonyms:
Watch what is "kinetics" in other dictionaries:
- (from Greek. Kinetikos leading in motion), the section of mechanics, mechanical is investigated in to rum. body condition due to physical. reasons by defining it. K. is divided into the dynamics of the doctrine of the movement of the body under the influence and statistics of the doctrine of equilibrium ... Physical encyclopedia
- (from Greek. Kinetikos leading in motion) Section of mechanics that combines statics and dynamics ... Large encyclopedic Dictionary
Kinetics, in physics one of the sections of the speaker. In chemistry, the section of physical chemistry considering the speed of chemical reactions. Studying the speed at different temperatures and pressure, chemists can determine how the reaction occurred ... Scientific and Technical Encyclopedic Dictionary
Kinetics, kinetics, mn. No, wives (from Greek. Kinetikos Motor) (fur.). Department of mechanics, hugging dynamics and statics. Explanatory dictionary of Ushakov. D.N. Ushakov. 1935 1940 ... Explanatory Dictionary Ushakov
- [NE], and, wives. Section of mechanics that combines statics and dynamics. | arr. Kinetic, Aya, Oe. Explanatory dictionary of Ozhegov. S.I. Ozhegov, N.Yu. Swedov. 1949 1992 ... Explanatory dictionary of Ozhegov
SUBS., Number of synonyms: 2 Macroeneetics (1) Psychokinetics (1) Dictionary of Synonyms ASIS. V.N. Trishin. 2013 ... Synonym dictionary
kinetics - Kinetics. Pronunciation [Kinetika] ... Dictionary of the difficulties of pronunciation and stress in modern Russian
kinetics - Teaching about the mechanism and speeds of physical. and chemical. processes. Physical. To. - The theory of non-equilibrium macroscopic. Processes in systems, concluded. From the state of thermal (thermodynamic) of equilibrium. To physical. k. Believe the thermodynamics of nonequilibrium ... ... Technical translator directory
KINETICS - (1) The physical times of the Affairs of theoretical physics, which studies the laws of processes arising in the system (gases, plasma, liquids, solid bodies) in its deviation from the state of thermodynamic equilibrium (for example, diffusion, thermal conductivity, ... ... Large polytechnic encyclopedia
Kinetics - The doctrine of the mechanism and speeds of physical and chemical processes. Physical kinetics The theory of non-equilibrium macro of the copies in systems derived from the state of thermal (thermodynamic) equilibrium. To physical kinetics ... Encyclopedic dictionary for metallurgy
Books
- Kinetics of photochemical reactions, I.S. Carpenters. Reproduced in the original author's spelling of the 1908 edition (publishing house "Moscow") ...
Page 1
As you know, the study of the interlocutor (partner of communication) according to his gestures, facial expressions and poses refers to the region of Kineyika. Consider only some of these kinematic components.
Worldwide, basic communication gestures do not differ from each other. When people are happy, they are smiling when they are sad - they frown, when they are angry - they have an angry look. Nod's head is almost all over the world means "yes" or approval. The gesture "Shoulders" is a good example of a universal gesture, which indicates that a person does not know or does not understand what is being in question.
As verbal languages \u200b\u200bdiffer from each other, depending on the type of culture, and the non-verbal language of one nation differs from the non-verbal language of another nation. It should be noted that the most common gesture is a touch, or tactile contact. Touch, or tactile contact, is the very first and most important in his life. Touching the mother shows not only physical well-being, but also expresses his love and tenderness. The child, devoid of this in childhood, lags behind the peers in intellectual development and acquires emotional defects that are almost impossible to compensate for in adulthood. Cultural norms significantly regulate tactile contacts. The touch remains a sign, first of all expressing feelings for a partner for communication. Rough, pain contacts accompany aggression and coercion. Soft, not pain contacts sign on trust and sympathy for a partner.
Most cultures impose many restrictions on touch. In each society there are ideas about how when, whom and who can touch. If you collect a list of touches, we will see that in different cultural layers they are carried out in different ways.
For example, a blow is an act of aggression, but the joking slam on the back, even very sensitive, old buddies perceived as a sign of a friendly location. In different cultures, the permissible number of touches differs significantly. So, in England, the interlocutors are very rarely touched upon each other. In Cambridge, the students were accepted to exchange hands twice a year - at the beginning and at the end school year. In countries Latin America, on the contrary, the frequency of touches is very large.
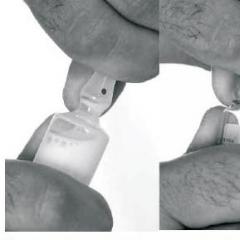
Handshake.An indispensable attribute of any meeting and farewell is a handshake. It can be very informative, especially its intensity and duration. Too short, sluggish handshake of very dry hands may indicate indifference. On the contrary, a prolonged handshake and too wet hands indicate a strong excitement. A little extended handshake along with a smile and a warm look demonstrates friendliness. However, to detain the hand of a partner in his hand is not worth it: he may have a feeling of irritation (he seemed to be in the trap).
You should consider the difference in views on the handshake of foreigners. For example, when a meeting with partners from Asia should not compress her palm too much and long. On the contrary, Western European and American entrepreneurs can not tolerate sluggish handshakes, since they are very accounted for athleticism and energy. They should be caught hand energetically and strongly.
With the help of various palm reversal, you can give this gesture various values. When your hand captures the hand of another person so that it turns out to be turned down his palm, is a powerful handshake. Such a handshake suggests that you want to dominate in the process of communicating with your partner.
When you stretch your hand, turning it out with my palm up, -
this is a submissive handshake. It is necessary in situations when you need to give the initiative to another person or let him feel the master of the situation.
A handshake in which the hands of the partner remain in the same position, indicates that both partners experience a sense of respect and mutual understanding.
A straight, not bent hand, like a powerful handshake, is a sign of disrespect. Its main purpose is to preserve the distance and remind of inequality.
The fire of finger tips is reminiscent of the fire, not bent hand, instead of hand, only fingers are in the palm. The purpose of the initiator of this handshake is to keep partners in communication at a convenient distance.
The gesture, called the Glove, means that his initiator is honest and he can trust. Such a gesture is applied only in relation to well familiar people. The fire with the use of both hands expresses the sincerity or depth of feelings towards the partner. For transmission of excess feelings, the left hand is used, which places on the right hand of the partner.
What science is studying the types of perception and why is it necessary? Is it only enough to shine in front of the erudine and knowledge of smart words? How to apply these knowledge in practice? All these questions arise every time we bump into the Internet spaces for tests to determine the type of perception. Is this a fashionable novelty that will soon forget? No, friends, there is no fresh flow.
What is the type of perception?
The first thoughts on the peculiarities of perception are found in the writings of antiquity philosophers. Approximately in the VI century. BC e. Thinking began to notice the difference in the perception of their students and describe their observations. These differences were interpreted in different ways, but the beginning was put.
It should be noted that until the XVIII century. The person was viewed by scientists as part of the society, which is understandable and logical. The approach to the study of the psychology of the personality and the development of the theory, which began to allow the principle of personal benefit from a person and evaluating all phenomena, based on their usefulness and acceptance by a separate individual, from the psychologists of Bentam and Smith. This moment became a turning point and finally turned the views of scientists in the right direction.
In the XIX-XX centuries. The development period began social psychology. The researchers first began to conduct laboratory experiments. It was this period that gave a clear understanding of the differences in the perception of people. The tests were created, the purpose of which was to determine the ways of perception by a person's information. Now the whole science is engaged in studying these subtleties, which is called "Socionics".
How do the types of perception are determined?
There are special tests. For the sake of curiosity, you have the opportunity to go through one of these tests directly on the Internet. Published a lot of books that tell about the types of perception including. As a rule, simple tests are printed in them, which, with some more likelihood, determine to which type of perception is closer. For people who set themselves the goal to deal with their abilities and peculiarities of perception, psychologists work. Tests on the type of perception that conducts a specialist is most reliable and comprehensive. From here, there is an absolutely logical question: "Why is it necessary?"
In order to understand the usefulness of these knowledge, it is necessary to recall the peculiarities of each type of perception and work with examples. First you need to say that pure types in terms of perception are extremely rare. We are talking about predisposition.
These people perceive the world in most cases through the eyes. It absolutely does not mean that visuals do not perceive sounds, odors and tactile sensations. For them, visual images bear more information and perceived better. So, you passed the test and identified our belonging to visuals. What's next? Use this feature in self-development. Each of us is learning. The need to absorb new information every day.
A person who mechanically performs already learned and communicated to the automatism of action begins to degrade. Children learn at school. How to help a small visual? Teach draw pictures during material development. Spectatical imageswhich are associated with certain information, he will remain forever. An adult visual must fulfill the instructions of the bosses, your career growth directly depends on this. Draw the scheme, it is this way that will help you understand how to most effectively fulfill the task.
These representatives of the human genus better perceive information on hearing. How to use it? Kids need to read out loud. Schoolchildren audies will learn better if the information is most filed not through the printed text, and orally. How to work an adult audio? Pronounce with the authorities and colleagues set up to your task. For you, a piece of paper with the instruction is less effective than direct communication. IN last years The audies were presented a magnificent gift - audio books. By the way, this is another way to identify your belonging to this type. Do you like this way of learning? Use on health!
These people more brightly perceive sensations, touch, experience. How to use it in everyday life? Perceive information emotionally and tie it to a certain feeling. You can explain your point of view for a long time for a long time, leading logical arguments, but you will not achieve anything. He needs to feel, feel and skip information through emotions. This feature must be used. Are you a kineette? Tie new knowledge with sensations that can be called in imagination.
Information is perceived through numbers, logical understanding, clear arguments. This category is rare. Although, to be fully honest, in recent years, psychologists noticed that people like this type began to be born more often. What is it connected with? There are no clear response from specialists. Tests determine the locality, but it is possible that these people represent a new stage of the evolution of mankind. How to live if you discrete? Search logic in all manifestations external world, Build chains, draw diagrams. This will help to understand the deep essence of incomprehensible and illogical, at first glance, things.
So, test yourself, friends. If you need to seriously deal with your abilities and learn how to use them with maximum benefit - please contact a psychologist. Tests for determining the peculiarities of perception are developed a lot, they will help you know yourself better.
Kinetics of biological processes - the teaching of the patterns and speeds of the flow of various biological processes (biochemical reactions, bioelectric phenomena, permeability through biological membranes, etc.); One of the directions of biophysical chemistry (see). Research results K. b. P. Find use in honey. practice. In particular, methods K. b. P. Used in the study of physical chemical. The mechanisms underlying the emergence of certain pathol, states, when establishing fiz.-chemical. Both Biohim, selection criteria or directed search for pharmacologically active substances (See Pharmacokinetics), when studying the dynamics of enzymatic processes occurring in the body normally and pathology.
At the heart of K. b. P. Lies the kinetics of chemical reactions that study the laws of the flow of Chem. Processes in time, their speed and mechanisms. Research in this area involves the main directions of development of the SCU, Chemistry and Chemical. Industry, Development of the principles of Him Management. processes, study of "behavior" of various chemicals. substances and products from them in various conditions, etc.
Chem. The reactions, as a result of which the starting materials are directly converted into the reaction products (i.e., flow into one stage), are called simple or single-sided reactions. If the process of converting the starting substances into reaction products proceeds into several stages, the reaction is called complex or multi-stage. The overwhelming majority of biochemical, reactions occurring in the body, is complex reactions.
Chem. The reactions can flow in a homogeneous system, i.e. within the same phase, or heterogeneous system, i.e. on the border of the phase section.
The velocity of a homogeneous reaction is defined as the amount of substance that reacted or the reaction for a unit of time per unit of volume. Consequently, the velocity of a homogeneous reaction (V) can be defined as a change in the concentration of one of the substances (ΔC), which is reacting or the reaction to the reaction per unit (Δt), under the conditions of consistency of the reaction system:
The minus sign shows that the concentration of the substance decreases with time.
With a decrease in the period of time (Δt), the ratio ΔC / ΔT is increasingly approaching the true reaction rate (v) at the moment (T):
In more general Chem speed. Reactions (α + βB \u003d γm + Nn), which comes with the participation of many components, can be expressed by the equation:
where SA, SV ... Cn -Concentration of each of the components at the moment, α, β ... n is the stoichiometric coefficients of these substances in the reaction equation, K is the proportionality coefficient, or speed constant.
Temperature dependence of the chemical speed. The reactions are generally expressed by the Arrhenius equation:

where k0 is the value constant for this reaction, E is the basis of the natural logarithm, E is the activation energy of this reaction, R is a universal gas constant, T is an absolute temperature.
From the Arrhenius equation, it follows that with an increase in temperature in arithmetic progression The reaction rate increases in geometric progression and the smaller E, the greater speed The reaction occurs (little reactions are known, the speed of which decreases with increasing temperature). The rate of enzymatic reactions increases with an increase in temperature only until the inactivation temperature of this enzyme is reached; With a further increase in temperature, the rate of enzymatic reaction decreases.
The absolute values \u200b\u200bof the speed constants are the most simple chemical. Reactions can be calculated based on the theory of transition (or activated complex) proposed in 1935 by Eiring (H. Eyring). In accordance with this theory, the reaction expressed, for example, by the equation: A + Sun - \u003d AB + C, flows through the stage of the formation of the activated complex (ABC), in K-ROM atom (molecule) in belongs equally and the original molecule ( Sun), and new - (AB). In the course of further reaction, the activated complex decomposes to the final products A B and C. The theory of the activated complex makes it possible to determine the most likely direction of the reaction and calculate the speed of its flow.
When studying the kinetics, Chem. reactions and clarify the mechanism of their flow great importance It has the establishment of stoichiometry (molecularity) of the reaction, i.e. the numbers of molecules involved in the elementary act of Him. reactions, and the order of the reaction, i.e. the form of the reaction equation, to-rye describes the course of this reaction in time.
Monomolecular reactions (transformation undergoes one molecule) can be represented general equation:
BUT<-> In or a.<-> B + D.
To this kind, reactions include isomeric transformations (for example, a cissometer into a transzymersometer) and some decomposition reactions (for example, the decomposition of iodine molecules per atoms when heated: i 2 \u003d 2i).
In bimolecular reactions, two molecules of substances are involved (eg, the decomposition of iodide hydrogen: 2Hi \u003d H 2 + I 2 or the washing of esters by alkali: Rcoor 1 + NaOH \u003d rcoona + R 1 OH).
If the reaction rate depends on the concentration of one source substance, the reaction is called the first order reaction. The reactions of the first order, in addition to the above-mentioned monomolecular reactions, include hydrolysis of esters in the presence of excess water, catalytic decomposition of hydrogen peroxide, etc. These reactions are distinguished by the fact that the same periods of the reacted substance correspond to the same periods of time. The speed of such reactions is proportional to the product of concentrations of two substances. These reactions are called second order reactions. Among biochemical, reaction processes more high order Do not meet.
Known Chem. reactions whose speed is constant and does not depend on the concentration of reactant substances. To such reactions, called zero-order reactions include many enzymatic processes at high concentrations of substrate, the process of denaturation of proteins on the surfaces of the phase separation, etc. For such reactions between the loss of the concentration of the reactant and the time there is direct proportionality.
A large number of complex chemical are known. processes. They distinguish parallel, consistent, conjugate, autocatalytic, chain and other reactions. With parallel reactions, the initial substances simultaneously join two (or more) reactions. For example, Bertolet Salt with moderate heating at the same time undergoes the decay in two directions:

Serial reactions can be represented by the general equation A -\u003e B -\u003e C -\u003e D; Substances in and d are intermediate reaction products. An example is the hydrolysis of polysaccharides, for example:
If one of the consecutive reactions proceeds slowly compared to others, the total speed of the process is determined by the speed of this slow reaction. The conjugate reactions proceed in accordance with the equations:
a) a + in -\u003e m
b) a + c -\u003e n
In this case, one of the reactions (a) proceeds only when, together with it, another reaction occurs (b). In other words, the reaction is induced by the reaction b. The substance A, common to both reactions, is called the actor, the substance in the acceptor and the substance with the inductor. For example, rr Indigo (The substance B) is not discolored with oxygen (L), but if it is added to the R-R-Rent of benzaldehyde (C), the latter is oxidized to the benzoic k-ta (N) and at the same time indigo is oxidized to the izatin (m).
Outocatalytic reactions are also related to complex reactions (see autocatalysis), reversible reactions (see chemical reactions) and chain reactions (see). The kinetics of all these reactions is expressed by rather complex mathematical equations, since most of the biohim, reactions occurring in the body relates to complex reactions.
The heterogeneous reaction rate is defined as the amount of the substance that reacted or the reaction for a unit of time per unit of the surface phase of the partition. If the value of the phase surface, the reaction occurs on the reaction, is difficult to measure (which takes place usually in practice), then the heterogeneous reaction rate is not referred to a unit of the surface, but to a unit of mass or phase volume.
The speed of heterogeneous reactions is closely related to the transference processes of the substance. In a heterogeneous reaction, three main stages can be isolated: the supply of reagents to the phase surface (stage A), chemical. Reaction on the surface of the phase (b) and removal of the reaction products from the phase surface (B).
With low energy activation, Chem. Reactions The heterogeneous reaction rate is mainly determined (limited) speeds as the same stages. With a high energy of the activation of Chem. Reactions The speed of the heterogeneous process is limited by the second stage (b).
Features of the flow of Chem. Reactions to biol, systems consider the kinetics of biological processes. Biological reactions are enzymatic processes occurring in a complex organized system. Such systems exchange with environmental Energy and substance, as a result of which they are called open systems (see thermodynamics). Open systems have a number of specific kinetic properties, of which the most important should be considered the possibility of establishing a dynamic stationary state in them, with a large value of many internal parameters of the system (eg, the concentrations of components) are stored constant for a certain period. This is because the processes of the inflow and the outflow of the substance mutually compensate each other, which is one of the conditions for maintaining homeostasis (see).
Another feature of the biol, processes are that in an open system, complex enzyme reactions (polyfermentation processes) have a property to carry out self-regulation, i.e., increase or decrease the speed due to activations or inhibition (braking) of the process with finite products or substances formed during this Reactions (see Biological System, Autoreguing). The speed and direction of such a process is often regulated by a relatively small number of such stages, which in this case can be considered control (or defining). In the case, for example, the totality of consecutive reactions may be the stages flowing at the lowest speed. At the same time, the change in the rate of one or another stage under the influence of an inhibitor or activator leads to a change in the rate of leakage of the entire biol process.
The rate of flow of reactions in a living organism is determined primarily by the enzymes of control stages (key enzymes) that can affect the properties of other enzymes and the structural conditions for the development of the reaction. Therefore, K. b. P. It should be considered as kinetics of a complex process, which is a set of conjugate, consecutive, parallel and other reactions.
The study of various simple reactions constituting the combination of biol, processes using the Him methods. Kinetics shows that the main kinetic patterns and their complete mathematical description are quite complicated and even in the case of the most simple combinations of elementary chemical. reactions. However, the study of the kinetics of the complex process is significantly simplified, if it is possible to dismember it at the stage, highly differ in a time scale - that is, fast and slow stages. For example, photo projects (see Photochemical reactions) can be divided into fast (photoexcitation) and slow (photo product formation) stage. In the case of the simplest enzymatic reaction, the rapid stage is the formation of the enzymesubstrate complex, and slow - the formation of the final product, etc.
The kinetics of the complex process are often studied by analyzing the behavior of one defining link (control stage), or the most slow, limiting the overall speed of the entire reaction (with its sequentially turned on in the chain), or the most quick, which is the main reaction (with parallel inclusion in its circuit) . Such a link is often an elementary process that combines the reversible and irreversible stage.
The element with along with conventional intermediate products can be unstable formation, the relative concentrations of which are small, but nevertheless determine the flow of the entire process (eg, activated complexes excited by the light of the molecule, etc.). The rate of irreversible (or poorly recognizable) stage of the process as a whole directly depends on the concentration of such transition states. The stationary intermediate concentration (C) is established and maintained due to the existence of a reversible stage of the process, which allows us to calculate the concentration of the active intermediate product through the constant of its dissociation, equilibrium with the source substrate and the concentration of this substrate.
Various biologists processes occur with the participation of various enzymes that accelerate the reactions. The enzyme (E), binding to the reaction substrate (S), forms an enzymesubstural activated complex (ES). The reaction occurs according to the scheme:

where P is the product of the reaction. The first stage is reversible, and the second is irreversible (or poorly recognized).
Enzymatic reactions have characteristic feature saturation with substrate. For small concentrations of the substrate, the rate of the enzymatic reaction increases in proportion to its concentration (first-order reaction), and at large concentrations it becomes constant (i.e. it goes into the reaction of zero order).
The dependence of the rate of the enzymatic reaction on the concentration of the substrate is described by the Michaelis - Modeten equation:
v \u003d (V * S) / (KM + S),
where V is the reaction rate, V is the maximum reaction rate, Km is a Mihaelis constant, numerically equal to the substrate concentration, with a reaction, the reaction rate reaches the value of 0.5 V (see enzymes).
It is necessary to take into account that the kinetics of many enzymatic reactions turns out to be more complex than this follows from the Michaelis equation, since several substrates can participate in an enzymatic reaction and several products can be formed, and the specific reversible or irreversible activators or inhibitors (reversible, forming dissociating with the enzyme Complexes, and irreversible, forming weeks associated complexes, cause changes in functions, enzyme groups). The reversible inhibitor can change the catalytic effect of the enzyme due to the interaction directly with its active center, thereby binding to the substrate. Another mechanism is possible when the inhibitor interacts with the enzyme molecule section, which is located outside the active center. In the first case, the substrate and inhibitor, responding only with a free enzyme, will compete for the active center (competitive inhibition) and form the complexes of the enzyme-substrate (ES) complexes and an enzyme inhibitor (EI). In the second case, non-competitive inhibition, the inhibitor interacts with the enzyme outside the active center, and the catalytic properties of the enzyme change due to the indirect effect on the reaction capacity of the center (through the so-called regulatory center). At the same time, the substrate and the enzyme no longer compete for the active center, but interact with the enzyme independently, forming, in addition to ES and EI complexes, tertiary complexes - enzyme - substrate - inhibitor (ESI). The ratios between the rate of enzymatic reaction (V) and the concentrations of the substrate and the inhibitor are studied: for competitive inhibition
and for non-competitive

where I is the concentration of the inhibitor, Ki - the constant of the dissociation of the complex enzyme-inhibitor, S is the concentration of the substrate, V is the maximum reaction rate, KM is the Mikhaelis constant.
Competitive and non-competitive inhibition of enzymatic reactions is widespread in biol, systems. As an example of non-competitive braking, the action of heavy metals ions (HG, AG), participating in the poisoning of certain sections of active centers (in particular SH-groups of enzymes).
The inhibitor can also be a substrate enzymatic reaction (substrate inhibition). In this case, with an increase in the concentration of the substrate, the reaction rate at first increases, and then, reaching the maximum value, starts to fall, and the process is inhibited. Such a kinetics of the process is a consequence of the interaction of the substrate molecules with an enzyme leading to education along with an active and inactive enzyme-substrate complex. An example of substrate inhibition can serve, in particular, the response of urease inhibition by high concentrations of urea.
Speaking of activation of enzymatic reactions, it should be noted that some enzymes require to manifest their activity participation in the reaction of certain non-protein substances. For example, activators can serve various ions or coherents of complex nature. Na +, k + ions are activated by ATP-AZU, phosphofructure, pyruvatkinase, Mn + - decarboxylase and peptidase. Activators can change the enzyme molecule, turning inactive protein into an active enzyme. Such, for example, activation of pepsinogen in pepsin or tripsinogen in trypsin.
The activating or inhibitory effect on the enzymatic process can have hydrogen ions. It is known that pH determines the degree of dissociation of ionic groups of enzyme. If the ionic group is included in the active center, then when changing the pH, a change in the binding of the substrate with the enzyme. Usually, the speed of the enzymatic reaction, depending on the pH of the medium, is expressed by a curve with a maximum covering a relatively narrow range of pH of the medium * so, the maximum enzymatic activity of pepsin is manifested at PH approx. 2, carboxylase - with pH approx. 5, amylases - with pH approx. 7. Therefore, violations in the regulation of processes responsible for maintaining the necessary pH values \u200b\u200bin this medium often entail a pathol, changes in enzyme activity, which is observed with some patol, states.
One of the essential factors affecting K. b. p., You should consider the temperature. When studying the effect of temperature on the rate of biol, processes are usually used by the concepts of the temperature coefficient of the reaction rate (Q10) and activation energy. The activation energy of most biol, processes lies within 5-20 kcal / mol; photochim. The processes have the value of e within 500-1100 cal / mol.
The theory of the activated complex and absolute reaction rates makes it possible to take effect on the rate of enzymatic reactions not only the temperature and energy of activation, but also a hysterical (spatial) factor that takes into account the configuration of the reacting molecules and the possibility of reaction in the collision of molecules with only certain areas. This factor enters the Arrhenius equation, along with
collision scrap, in the constant K0. It is especially important to take into account it in the reactions of the interaction of large molecules. This makes it possible, in particular, to understand why some processes proceed very quickly even at low temperatures, despite the fact that the activation energy determined by temperature dependence is large. For example, with a pepsin denaturation, a smaller part of the energy barrier is determined by the energy, the K-Rui must be expected to break molecular bonds during the activation of pepsin (approx. 18 kcal / mol), and most of the steric factor (approx. 45 kcal / mol).
The reaction may play an important role in the body, during which products are formed, the catalysts themselves for the reaction themselves; Such reactions are called autocatalytic. For such a reaction, the speed depends on the concentration of both the initial and finite substances. The dependence of the change in the concentration of the final product in time is expressed in this case of a 5-shaped curve showing that the yield of the product during the initial period of time (induction period) is relatively small, and then the reaction rate increases sharply and the product will quickly accumulate.
Of particular interest are the so-called. Chain reactions. Initial activation (initiation) chain reactions It is carried out by free radicals (see radicals, chain reactions). Apparently, these reactions are developing in biol, systems (mainly in the membrane cell structures) with radiation lesions of the body or the action of some poisonous substances. The chain reactions of lipid oxidation in membranes lead to the rapid development of patol, processes associated with the destruction of cellular structures. The characteristic property of chain reactions of lipid oxidation in biol, membranes is the branching of oxidation chains. Branched chain reactions in time are developing on an S-shaped curve. The rate of chain reactions drops sharply under the action of substances reacting with free radicals, which leads to the cliff of the chains of oxidation and braking of the process (see Antioxidants). This is associated with the protective role of radical inhibitors in the reaction of radiation damage (see radiation damage).
A huge number of processes catalyzed by enzymes in a living cell is a clearly organized spatial-temporal "grid" of interconnected reactions (see metabolism and energy). In such a complex system, separate real biihim, reactions are flowing open systems, the speed of the process in which is determined mainly by the admission of the substrate and the outflow of final products. Such open systems are either in a stationary state or in the process of transition from one stationary state to another under the influence of any external factors. The transition from one stationary state to another is characterized by nonstationary kinetics. In this case, the appearance of periodic modes (autocolates of concentrations of reagents) is possible. Such processes may occur, in particular, while inhibiting the enzyme by the substrate and the product of the reaction. Oscillatory phenomena and periodic modes are well studied for glycolysis processes (see), photosynthesis dark reactions (see), various types of enzymatic reactions (see enzymes) for biological rhythms (see), ecole, systems (see Ecology) for nervous processes (see Nervous Impulse), etc.
The study of the kinetics of complex systems, k-fish and are biol, processes, often requires the use of the method of mathematical modeling (see), which allows you to describe the change in the basic parameters of biol, systems and time processes. There are special mathematical techniques that allow us to dismember the complex set of reactions to individual stages that differ in their speeds and study the behavior of the system without an accurate analytical solution. complex equations. This approach is successfully used to study the features of the flow of many biologists, processes.
Mathematical models have been developed for some enzymatic reactions, models of active biol, membranes (see Biological membranes), the growth of cell populations, the dynamics of immune reactions, etc., were developed, and others. With the help of a mathematical model of the immune response, in particular, the nature of periodic modes reflecting the oscillatory course of some Malaria diseases. With the help of mathematical modeling, the dynamics of the primary and secondary immune response was also successfully studied.
Study K. b. P. With the help of methods of mathematical modeling makes it possible to obtain information about the essence of the studied phenomena and use the results in practice. The study of the kinetics of inhibition and activation of reactions, the kinetics of the permeability of biol, the cell membranes, the study of the rates of reactions responsible for the elimination of certain substances from the body, are of great importance in solving the issue of pharmacol, the values \u200b\u200bof one or another drug or the nature of its action in various conditions ( See Pharmacokinetics). Variations of patterns due to changes in temperature conditions, pH values, the nature of regulation and other parties K. b. n., are associated with a feature of the activities of many of the most important enzymes and can serve as information on the development of various pathol, processes. The growth dynamics of various neoplasms into the organic neck, acceleration or braking of the process under the influence of various internal and external factors is also investigated using kinetic methods for analyzing biol * processes.
Bibliography: Berezin I. V. and Klesov A. A. Practical course Chemical and Enzymatic Kinetics, M., 1976; Biophysics, ed. B. N. Tusarova and O. R. Necklace, p. 48, M., 1968; Eremin E. N. Basics of Chemical Kinetics, M., 1976, Bibliogr.; Kursk MD, Kosty-r and N S. A. and P s b a l h E nk about V. K. Biochemical kinetics, Kiev, 1977, bibliogr.; Leninger A. Biochemistry, Per. from English, M., 1976; Romanovskiy Yu. M., Stepanova N. V. I''ler, N A V to K and Y D. S. Math modeling in biophysics, M., 1975, bibliogr.; Rubin A. B. Thermodynamics of biological processes, M., 1976; Wait N. Chemical Kinetics, Per. from English, M., 1974; Westley J. Enzymatic Catalysis, Per. from English, M., 1972; Emanuel H. M. Kinetics of experimental tumor processes, M., 1977; Emanuel H. M. Iknorra D. G. Course of Chemical Kinetics, M., 1974.
B. A. Glyaev; V.P. Mishin (Chem. Kinetics).