Distance between molecules in solid liquid. The interaction of molecules
We are solid called such substances that are capable of forming the bodies and have a volume. They differ from liquids and gases with their shape. Solid substances retain the body shape due to the fact that their particles are not able to move freely. They differ in their density, plasticity, electrical conductivity and color. They also have other properties. For example, most of these substances melt during heating, purchasing a liquid aggregate state. Some of them, when heated, immediately turn into gas (derived). But there are also those that decompose on other substances.
Types of solid substances
All solids are divided into two groups.
- Amorphous, in which individual particles are chaotic. In other words: they do not have a clear (defined) structure. These solids are able to melt in some installed temperature range. The most common of them can include glass and resin.
- Crystal, which, in turn, are divided into 4 types: atomic, molecular, ionic, metal. In them, particles are located only according to a specific scheme, namely in nodes crystal lattice. Its geometry in different substances can vary greatly.
Solid crystalline substances prevail over amorphous by their number.
Types of crystalline solids
In solid state, almost all substances have a crystal structure. They differ in their lattice in their nodes contain various particles and chemical elements. It was according to them that they received their names. Each type has characteristic properties for it:
- In the atomic crystal lattice, the particles of the solid are associated with a covalent bond. It is distinguished by its strength. Due to this, such substances are high and boiling. This type includes quartz and diamond.
- In the molecular crystal lattice, the connection between particles is distinguished by its weakness. Substances of this type are characterized by ease of boiling and melting. They differ in volatility, thanks to which they have a certain smell. Such solid bodies include ice, sugar. Movement of molecules in solids of this type is characterized by its activity.
- In the nodes alternate the corresponding particles charged positively and negatively. They are held by electrostatic attraction. This type of lattice exists in alkali, salts, many substances of this species are easily dissolved in water. Due to the sufficiently strong connection between the ions they are refractory. Almost all of them do not smell, because it is characterized by non-volatile. Substances with ion lattice are unable to conduct electricitybecause there are no free electrons in their composition. Typical example ion solid - Cooking salt. Such a crystal lattice gives her fragility. This is due to the fact that any shear can lead to the emergence of the pushing forces of ions.
- Only ions are present in the metal crystal lattice chemical substancesCharged positively. Between them there are free electrons through which thermal and electrical energy is perfect. That is why any metals are distinguished by such a feature as conductivity.

General concepts about solid
Solid bodies and substances are almost the same thing. These terms call one of 4 aggregate states. Solid bodies have a stable shape and nature of the thermal motion of atoms. Moreover, the latter make small oscillations next to equilibrium positions. The section of science involving the composition and internal structure is called solid physics. There are other important areas of knowledge engaged in such substances. The change in shape with external influences and movement is called the mechanics of the deformable body.
Due to different properties of solids, they found use in different technical devices created by man. Most often, their use was based on properties such as hardness, volume, mass, elasticity, plasticity, fragility. Modern science allows the use of other quality solids that can be detected solely in laboratory conditions.
What is crystals
Crystals are solid bodies with particles located in a certain order. Each complies with its structure. Its atoms form three-dimensional-periodic laying, called a crystal lattice. Solid substances have different symmetry of the structure. The crystalline state of the solid is considered stable, since it has a minimum amount of potential energy.
The overwhelming majority of solid consists of a huge number of randomly oriented individual grains (crystallites). Such substances are called polycrystalline. These include technical alloys and metals, as well as many mountain breeds. Monocrystalline call single natural or synthetic crystals.
Most often, such solid bodies are formed from the state of the liquid phase represented by the melt or solution. Sometimes they are also obtained from a gaseous state. This process is called crystallization. Thanks to the scientific and technical progress, the process of growing (synthesis) of various substances has received an industrial scale. Most of the crystals have natural shape In the form of their dimensions are very different. So, natural quartz (rhinestone) can weigh up hundreds of kilograms, and diamonds - up to several grams.

In amorphous solid telah atoms are in constant fluctuations around chaotic points. They maintain a certain neighboring order, but there is no far. This is due to the fact that their molecules are located at a distance that can be compared with their size. The most common example of such a solid in our lives is a glassy state. Often are considered as a liquid with an infinitely high viscosity. The time of their crystallization is sometimes so great, which is not manifested at all.
It is the above properties of these substances make them unique. Amorphous solid bodies are considered unstable, since over time they can go to the crystalline state.
Molecules and atoms from which the solid consists, packed with a large density. They practically retain their realization relative to other particles and are held together due to intermolecular interaction. The distance between the solid molecules in different directions is called the crystal lattice parameter. The structure of the substance and its symmetry is determined by many properties, such as an electronic zone, spheel and optics. When exposed to a solid, sufficiently large force, these qualities can be violated to one degree or another. In this case, the solid is amenable to residual deformation.
The atoms of solid bodies perform oscillatory movements, which are due to the possession of them by thermal energy. Because they are negligible, they can only be observed under laboratory conditions. The solid is largely affected by its properties.

Study of solids
Features, properties of these substances, their quality and movement of particles are studied by various subsections of solid physics.
For research used: radio spectroscopy, structural analysis using X-ray and other methods. So the mechanical, physical and thermal properties of solids are studied. Hardness, resistance to loads, tensile limit, phase transformations are studied by materials. It is largely echoing with solid physics. There is another important modern science. The study of existing and synthesizing new substances is carried out by chemistry of solid state.
Features of solids
The nature of the movement of the external electrons of solids atoms determines many of its properties, for example, electrical. There are 5 classes of such bodies. They are established depending on the type of bonding of atoms:
- Ionic, the main characteristic of which is the power of electrostatic attraction. Its features: reflection and absorption of light in the infrared area. At low temperatures, the ionic communication is characterized by small electrical conductivity. An example of such a substance is the sodium salt of hydrochloric acid (NaCl).
- Covalent, carried out at the expense of an electronic pair, which belongs to both atoms. Such a connection is divided into: single (simple), double and triple. These names are talking about the presence of steam of electrons (1, 2, 3). Double and triple ties are called multiple. There is another division of this group. So, depending on the distribution of electron density, polar and non-polar communications are isolated. The first is formed by different atoms, and the second one is the same. Such a solid state of substance, examples of which - diamond (C) and silicon (Si) differs in its density. The solid crystals belong to the covalent bond.
- Metal, formed by combining the valence electrons of atoms. As a result, a general electronic cloud occurs, which shifts under the influence of electrical stress. Metal communication is formed when the binded atoms are large. They are able to give electrons. Many metals and complex compounds this bond forms a solid state of matter. Examples: Sodium, Barium, Aluminum, Copper, Gold. From non-metallic compounds, the following can be noted: AlCr 2, Ca 2 Cu, Cu 5 Zn 8. Metal bond substances (metals) are diverse in physical properties. They can be liquid (HG), soft (Na, k), very solid (W, NB).
- Molecular arising in crystals, which are formed by individual substance molecules. It is characterized by gaps between molecules with zero electron density. Forces binding atoms in such crystals are significant. At the same time, molecules are attracted to each other only with a weak intermolecular attraction. That is why the relationship between them is easily destroyed when heated. The compounds between atoms are destroyed much more difficult. Molecular communication is divided into orientational, dispersion and induction. An example of such a substance is solid methane.
- Hydrogen, which occurs between positively polarized molecule atoms or its part and a negatively polarized smallest particle of a different molecule or other part. Such connections include ice.

Properties of solids
What do we know today? Scientists have long been studying the properties of the solid state of the substance. When exposed to temperatures, it changes and it changes. The transition of such a body into liquid is melted. The transformation of the solid in the gaseous condition is called sublimation. When the temperature decreases, the solid crystallization occurs. Some substances under the influence of cold are moving into the amorphous phase. This process scientists are called glass.
When the internal structure of solids changes. It acquires the greatest orderliness with a decrease in temperature. At atmospheric pressure and temperature T\u003e 0 to any substances that exist in nature are solidified. Only helium, for the crystallization of which the pressure of 24 atm is needed, makes up an exception to this rule.
The solid state of the substance gives it various physical properties. They characterize the specific behavior of the bodies under the influence of certain fields and forces. These properties are divided into groups. 3 methods of exposure corresponding to 3 types of energy (mechanical, thermal, electromagnetic) are isolated. Accordingly, there are 3 groups of physical properties of solids:
- Mechanical properties associated with voltage and deformation of tel. For these criteria, solids are divided into elastic, rheological, strength and technological. At rest, such a body retains its shape, but it may vary under the action of external force. At the same time, its deformation can be plastic (the initial species is not returned), elastic (returns to the original form) or destructive (when a certain threshold is reached, the decay / spill occurs). Feedback on the attached force is described by modulosions of elasticity. The solid body resists not only compression, stretching, but also shifts, cruel and bending. The strength of the solid is called its property to resist destruction.
- Thermal, manifested when exposed to thermal fields. One of the most important properties is the melting point at which the body goes into a liquid state. It is noted in crystalline solids. Amorphous bodies have a hidden heat of melting, since their transition to a liquid state with increasing temperature occurs gradually. Upon reaching a certain heat, the amorphous body loses its elasticity and acquires plasticity. This condition means achieving the glass transition temperature. When heated, a solid deformation occurs. And it is most often expanding. Quantitatively, this condition is characterized by a certain coefficient. The body temperature affects the mechanical characteristics such as fluidity, plasticity, hardness and durability.
- Electromagnetic, associated with the impact on the solid of the flows of microparticles and electromagnetic waves of high rigidity. They are conditionally related to radiation properties.

Zone structure
Solids are classified and for the so-called zone structure. So, among them distinguish:
- Explorer, characterized in that the zones of their conductivity and valence overlap. In this case, electrons can move between them, getting the slightest energy. Conductors include all metals. When the potential difference is applied to this body, an electric current is formed (due to the free movement of electrons between points with the smallest and large potential).
- Dielectrics whose zones do not overlap. The interval between them exceeds 4 eV. To carry out electrons from the valence in the area, a large energy is necessary. Thanks to these properties, the dielectrics almost do not conduct current.
- Semiconductors characterized by the absence of conduction zones and valence. The interval between them is less than 4 eV. To transfer electrons from the valence to the area, an energy is needed smaller than for dielectrics. Clean (illegal and eigenvalued) semiconductors are poorly skipping current.
Movement of molecules in solids cause their electromagnetic properties.
Other properties
Solid bodies are divided into their magnetic properties. There are three groups:
- Diamagnetics whose properties are little depend on temperature or aggregate state.
- Paramagnetics, which are a consequence of the orientation of the conductivity electrons and magnetic moments of atoms. According to the Curi law, their susceptibility decreases in proportion to temperature. So, at 300 K it is 10 -5.
- Bodies with an ordered magnetic structure with distant order of atoms. In the nodes of their grille, particles with magnetic moments periodically dispose. Such solid bodies and substances are often used in different spheres of human activity.

Solid solids in nature
What are they? The density of solids largely determines their hardness. Per last years Scientists have discovered several materials that claim the title of "the most durable body". The hardest substance is a fullerti (crystal with fullerene molecules), which is about 1.5 times hard with diamond. Unfortunately, it is still available only in extremely small quantities.
Today, the solid substance, which in the future, may be used in industry, - Lonsdaleit (hexagonal diamond). It is 58% of the harder diamond. Lonsdaleit is allotropic carbon modification. Its crystal lattice is very similar to diamond. The LONSDELIT cell contains 4 atoms, and a diamond - 8. From the widely used crystals, the most solid remains diamond.
Physics. Molecules. Location of molecules in a gaseous, liquid and solid distance.
- In the gaseous state of the molecule is not connected with each other, are at a high distance from each other. Movement Brownian. Gas can be relatively easy to compress.
In liquid - molecules close to each other, fluctuate together. Almost do not give in compression.
In Firdom - molecules are strict (in crystalline lattices), there is no molecules. Compression does not succumb. - The structure of the substance and the start of chemistry:
http://samlib.ru/a/anemow_e_m/aa0.shtml
(without registration and SMS messages, in a handy text format: You can use Ctrl + C) - It is impossible to agree with the fact that the molecule is not moving in the solid state.
Movement of molecules in gases
In gases, the distance between molecules and atoms is significantly larger than the dimensions of molecules, and attraction forces are very small. Therefore, gases do not have their own form and constant volume. Gases are easily compressed because repulsion forces on large distances Also small. Gaza possess the property to unlimited expanding, filling the entire volume provided to them. Gas molecules are moving with very big speeds, encounter each other, bounce apart from each other in different directions. Numerous blows of molecules about the wall of the vessel create gas pressure.
Movement of molecules in liquids
In liquids, the molecule not only fluctuate near the position of the equilibrium, but also make a jump from one equilibrium position in the neighboring. These jumps occur periodically. The time segment between such jumps was the name of the average time of the settling life (or average relaxation time) and is indicated by the letter?. In other words, relaxation time is the time of oscillations about one particular equilibrium position. At room temperature, this time is an average of 10-11 seconds. The time of one oscillation is 10-1210-13 s.
The time of classroom life decreases with an increase in temperature. The distance between liquid molecules is less than the dimensions of molecules, the particles are located close to each other, and the intermolecular attraction is large. Nevertheless, the location of fluid molecules is not strictly ordered throughout the volume.
Fluids, like firm bodies, retain their volume, but do not have their own form. Therefore, they take the shape of the vessel in which there are. The liquid has such a property as fluidity. Due to this property, the liquid does not resist the change in the shape, it is slightly compressed, and the physical properties are the same in all directions inside the liquid (isotropy of liquids). For the first time, the nature of the molecular movement in fluids was established by Soviet physicist Yakov Ilyich Frenkel (1894 1952).
Movement of molecules in firm bodies
Molecules and firm body atoms are located in a certain order and form a crystal lattice. Such solids are called crystalline. Atoms make oscillatory movements near the position of the equilibrium, and the attraction between them is very large. Therefore, firm bodies in normal conditions retain the volume and have their own forms
- In gaseous-moving randomno, drove
In liquid moving in accordance with each other
In solid - do not move.
liquids, amorphous and crystalline bodies
gas and liquids
gases, liquids and crystalline bodies
approximately equal to the diameter of the molecule
less diameter of the molecule
about 10 times larger than the diameter of the molecule
depends on gas temperature
liquids
crystal tel
amorphous tel
only models of the structure of gases
only models of the structure of amorphous bodies
models of the structure of gases and liquids
models of the structure of gases, liquids and solids
the distance between molecules increases
molecules begin to attract each other
increases orderliness in the location of molecules
the distance between molecules is reduced
not changed
increased 5 times
decreased 5 times
increased to the root of five times
Distances between molecules are comparable with dimensions of molecules (under normal conditions) for
In the gases under normal conditions, the average distance between molecules
The smallest orderliness in the location of the particles is characteristic of
The distance between adjacent particles of the substance is on average many times more than the dimensions of the particles themselves. This statement corresponds to the model
In the process of transition of water from liquid state in crystal
At constant pressure, the concentration of gas molecules increased 5 times, and its mass changed. The average kinetic energy of the progressive movement of gas molecules
The table shows the melting and boiling temperatures of some substances:
substance | Boiling temperature | substance | Melting temperature |
naphthalene |
Choose a true statement.
Melted Melted Melting Melting Mus for Ether Boil
The boiling point of alcohol is less than mercury melting temperature
The boiling point of the alcohol is larger than the melting point of naphthalene
Boiling point of ether less naphthalene melting temperature
The solid temperature dropped 17 ºС. In the absolute scale of temperatures, this change was
1) 290 K 2) 256 K 3) 17 K 4) 0 K
9. In the vessel of the constant volume there is ideal gas in the amount of 2 mol. How to change the absolute temperature of the gas vessel temperature when the vessel is released 1 mol of gas so that the gas pressure on the vessel walls has increased by 2 times?
1) increase by 2 times 3) increase by 4 times
2) reduce 2 times 4) reduce 4 times
10. At temperature T and pressure p, one mol of perfect gas occupies V. What is the volume of the same gas taken in an amount of 2 mol, at a pressure of 2p and 2T temperature?
1) 4V 2) 2V 3) v 4) 8v
11. The temperature of the hydrogen, taken in the amount of 3 mol, in the vessel is T. What is the temperature of oxygen taken in the amount of 3 mol, in the volume of the same volume and at the same pressure?
1) T 2) 8T 3) 24 t 4) T / 8
12. In the vessel, closed piston, is the perfect gas. A graph of the pressure of gas pressure on temperature with changes in its condition is shown in the figure. What gas state corresponds to the smallest value volume?
1) A 2) in 3) with 4) D
13. In a constant volume vessel, the perfect gas is changed, the mass of which is changed. The diagram shows the process of changing the state of the gas. In which points of the chart the mass of the gas is the greatest?
1) A 2) in 3) with 4) D
14. At the same temperature, the saturated steam in the closed vessel differs from unsaturated steam in the same vessel
1) pressure
2) Movement speed of molecules
3) the average energy of the chaotic movement of molecules
4) the lack of impurities of foreign gases
15. What point in the diagram corresponds to the maximum gas pressure?
you can not give an accurate answer
17. Balloon of 2500 cubic meters. With a mass of the shell of 400 kg, there is a hole at thenime, through which the air in the ball is heated by the burner. To which minimum temperature you need to heat the air in a bowl so that the ball take off together with the cargo (basket and aeronaut) weighing 200 kg? The ambient temperature is 7ºС, its density is 1.2 kg per cubic meters. The bowl of the ball is considered a negress.
MTC and thermodynamics
MTC and thermodynamics
According to this section, five tasks with the choice were included in each option.
answer from which 4 is the base level and 1 - elevated. According to the exam results
the following content elements were learned:
The use of the Mendeleev-Klapairone equation;
The dependence of the gas pressure on the concentration of molecules and temperature;
The amount of heat during heating and cooling (calculation);
Features of heat transfer;
Relative humidity of air (calculation);
Work in thermodynamics (chart);
The use of the equation of the state of the gas.
Among the tasks of the basic level, the following questions caused:
1) change in internal energy in various isoproces (for example,
isoormal increase in pressure) - 50% of execution.
2) Graphics of Isoprocesses - 56%.
Example 5.
The constant weight of the perfect gas is involved in the process shown
on the image. The greatest gas pressure in the process is achieved
1) at point 1
2) on the whole segment 1-2
3) at point 3
4) on the whole segment 2-3
Answer: 1.
3) Determination of air humidity - 50%. These tasks contained a photo
psychrometer, according to which it was necessary to remove the testimony of dry and wet
thermometers and then identify air humidity using part
the psychrometric table shown in the task.
4) Application of the first law of thermodynamics. These tasks were the most
complicated among the tasks of the baseline under this section - 45%. Here
it was necessary to take advantage of the schedule, determine the type of isoprocess
(used either isotherms or isoohra) and in accordance with this
determine one of the parameters according to the specified one.
Among the tasks increased level The calculated tasks were presented on
the use of a gas state equation with which an average of 54% has coped
students, as well as previously used tasks for changing changes
the parameters of the ideal gas in an arbitrary process. With them successfully copes
only a group of strong graduates, and the average percentage of execution was 45%.
One of these tasks is shown below.
Example 6.
In the vessel, closed piston, is the perfect gas. Process
changes in the state of the gas is shown in the diagram (see Figure). how
changed the volume of gas during its transition from the state and in state in?
1) all the time increased
2) all the time decreased
3) first increased, then decreased
4) first decreased, then increased
Answer: 1.
Types of activity number
% tasks
photos2 10-12 25.0-30.0
4. Physics
4.1. Characteristics of control measuring materials in physics
2007.
Examination for a single state exam in 2007 had
the same structure as for the two previous years. It consisted of 40 tasks,
differing imaging form and level of complexity. In the first part of the work
30 tasks with a response choosing where each task was driven
four answers, from which only one was correct. The second part contained 4
tasks with a brief answer. They were estimated tasks after the decision
which required a response in the form of a number. The third part of the examination
work is 6 settlement tasks to which it was necessary to bring full
deployed solution. The total work time was 210 minutes.
Education and Specification Item Codifier
examination were compiled on the basis of a mandatory minimum
1999 No. 56) and took into account the federal component of the State Standard
medium (full) education in physics, profile level (Order MO from 5
march 2004 No. 1089). Content elements codifier did not undergo changes
compared since 2006 and included only those elements that simultaneously
are present as a federal component of the State Standard
(profile level, 2004) and in the mandatory minimum content
education 1999
Compared with the control measuring materials 2006 in options
EGE 2007 was made two changes. The first of them was redistributed
tasks in the first part of the work on the thematic basis. Regardless of difficulty
(basic or elevated levels), first followed all tasks for mechanics, then
on MTC and thermodynamics, electrodynamics and, finally, according to quantum physics. Second
change concerned the purposeful introduction of tasks inspecting
forgetting methodological skills. In 2007, the A30 tasks checked the skills
analyze the results of experimental studies expressed in the form
tables or graphics, as well as build graphs according to the results of the experiment. Selection
the tasks for the A30 line were carried out on the basis of the need for verification in this
series of options for one type of activity and, accordingly, regardless of
thematic affiliation of a specific task.
In the examination work, the tasks of the basic, elevated
and high levels of difficulty. The tasks of the baseline tested the assimilation of the most
important physical concepts and laws. Advanced levels controlled
the ability to use these concepts and laws for analyzing more complex processes or
the ability to solve the task of applying one or two laws (formulas) for any of
those school courses of physics. The tasks of the high level of complexity are calculated
objectives that reflect the level of requirements for entrance exams in universities and
require the use of knowledge at once from two or three sections of physics in a modified or
new situation.
In Kim 2007, tasks were included in all major meaningful
sections of the course of physics:
1) "Mechanics" (kinematics, dynamics, static, laws of conservation in mechanics,
mechanical oscillations and waves);
2) "Molecular physics. Thermodynamics";
3) "Electrodynamics" (electrostatics, d.C., a magnetic field,
electromagnetic induction, electromagnetic oscillations and waves, optics);
four) " The quantum physics"(Elements of a hundred, corpuscular wave dualism, physics
atom, physics of the atomic nucleus).
Table 4.1 shows the distribution of tasks on the blocks of content in each
from parts of examination work.
Table 4.1.
depending on the type of tasks
All work
(with a choice
(with short
quests% count
quests% count
% tasks
1 mechanics 11-131 27.5-32,5 9-10 22.5-25.0 1 2.5 1-2 2.5-5.0
2 MTCs and thermodynamics 8-10 20.0-25.0 6-7 15.0-17.5 1 2.5 1-2 2.5-5.0
3 Electrodynamics 12-14 30.0-35,5 9-10 22.5-15.0 2 5.0 2-3 5.0-7.5
4 quantum physics and
One hundred 6-8 15.0-20.0 5-6 12.5-15.0 - - 1-2 2.5-5.0
Table 4.2 shows the distribution of tasks on blocks of content in
depending on the level of complexity.
Table4.2
Distribution of tasks by courses of physics
depending on the level of complexity
All work
A basic level of
(with a choice
Increased
(with a choice of response
and short
High level
(with deployed
Response section)
quests% count
quests% count
quests% count
% tasks
1 mechanics 11-13 27.5-32.5 7-8 17.5-20.0 3 7.5 1-2 2.5-5.0
2 MT and thermodynamics 8-10 20.0-25.0 5-6 12.5-15.0 2 5.0 1-2 2.5-5.0
3 Electrodynamics 12-14 30.0-35.5 7-8 17.5-20.0 4 10.0 2-3 5.0-7.5
4 quantum physics and
One hundred 6-8 15.0-20.0 4-5 10.0-12.5 1 2.5 1-2 2.5-5.0
When developing the content of the examination work, took into account
the need to verify mastering various activities. Wherein
the tasks of each of the series of options were chosen taking into account the distribution by type
activities presented in Table 4.3.
1 Changing the number of tasks for each of themes is associated with various themes of complex problems C6 and
a30 assignments that inspect the methodological skills on the material of different sections of physics, in
different series of options.
Table4.3
Distribution of tasks by type of activity
Types of activity number
% tasks
1 Understand the physical meaning of models, concepts, quantities 4-5 10.0-12.5
2 Express physical phenomena, distinguish between different
factors on the flow of phenomena, manifestations of phenomena in nature or
their use in technical devices and everyday life
3 Apply the laws of physics (formulas) to analyze the processes on
quality level 6-8 15.0-20.0
4 Apply the laws of physics (formulas) to analyze the processes on
estimated level 10-12 25.0-30.0
5 Analyze the results of experimental studies 1-2 2.5-5.0
6 Analyze information obtained from graphs, tables, schemes,
photos2 10-12 25.0-30.0
7 Solve problems of various levels of complexity 13-14 32.5-35.0
All tasks of the first and second parts of the examination work were estimated at 1
primary score. Solutions to the tasks of the third part (C1-C6) were checked by two experts in
accordance with the generalized evaluation criteria, taking into account the correctness and
full response. Maximum score For all tasks with a detailed response ranged 3
point. The task was considered to be solved if the student scored at least 2 points for it.
Based on the points issued for the execution of all tasks of the examination
works, translated into "test" scores on a 100-point scale and in marks
on a five-point scale. Table 4.4 reflects the ratios between primary,
test marks on the five-point system over the past three years.
Table4.4
Ratio primary ballots , test points and school marks
Years, scores 2 3 4 5
2007 primary 0-11 12-22 23-35 36-52
tests 0-32 33-51 52-68 69-100
2006 Primary 0-9 10-19 20-33 34-52
tests 0-34 35-51 52-69 70-100
2005 Primary 0-10 11-20 21-35 36-52
tests 0-33 34-50 51-67 68-100
Comparison of primary ballov boundaries shows that this year the conditions
obtaining the corresponding marks were more stringent compared to 2006, but
approximately corresponded to the conditions of 2005. It was due to the fact that in the past
year single exam In physics, not only those who were going to enter universities
according to the appropriate profile, but also almost 20% of students (from the total number of passing),
who studied physics on basic level (For them, this exam was by decision
region mandatory).
In total, 40 options were prepared for the exam in 2007,
which were five episodes of 8 options created by different plans.
Series of options differed in controlled elements of content and species
activities for the same line of tasks, but in general they all had about
2 In this case, there is a form of information presentation in the text of the task or distractors,
therefore, the same task can check two types of activity.
same average level complexity and corresponded to the examination plan
the work shown in Appendix 4.1.
4.2. Characteristics of participants of the exam in physics2007 of the year
The number of participants of the exam in physics this year amounted to 70,052 people that
significantly lower than in the previous year, and approximately corresponds to the indicators
2005 (see Table 4.5). The number of regions in which graduates handed over the EE
physics, increased to 65. The number of graduates who choose physics in format
EGE is significantly different for different regions: from 5316 people. In the Republic
Tatarstan up to 51 people. in Nenets autonomous District. In percentage of
the total number of graduates the number of participants of the exam in physics varies from
0.34% in Moscow to 19.1% in the Samara region.
Table4.5
Number of exam participants
Year the day of the girl youth
regions
participants Number% number%
2005 54 68 916 18 006 26,1 50 910 73,9
2006 61 90 3893 29 266 32,4 61 123 67,6
2007 65 70 052 17 076 24,4 52 976 75,6
Physics exam choose mainly young men, and only a quarter from
the total number of participants make up girls who have chosen to continue
formation of university physico-technical profile.
Almost no year from year to year and the distribution of exam participants
types of settlements (see Table 4.6). Almost half of graduates who surrendered
Exam in physics lives in major cities and only 20% are students who ended
rural schools.
Table4.6
Distribution of the participants of the exam in the types of settlements, in which
their educational institutions are located
The number of examinations percentage
Type of populated area examined
Settlement of rural type (village,
village, Khutor, etc.) 13 767 18 107 14 281 20.0 20.0 20.4
City type
(Working settlement, urban village
type, etc.)
4 780 8 325 4 805 6,9 9,2 6,9
City with a population less than 50 thousand people 7 427 10 810 7 965 10.8 12.0 11,4
City with a population of 50-100 thousand people 6 063 8 757 7 088 8.8 9.7 10.1
City with a population of 100-450 thousand people 16 195 17 673 14 630 23,5 19.5 20.9
City with a population of 450-680 thousand people 7 679 11799 7 210 11.1 13.1 10.3
City with a population of more than 680 thousand
man 13 005 14 283 13 807 18.9 15.8 19.7
st. Petersburg - 72 7 - 0.1 0.01
moscow - 224 259 - 0.2 0.3
No data - 339 - - 0.4 -
Total 68 916 90 389 70 052 100% 100% 100%
3 in 2006 in one of the regions entry exams In universities in physics were carried out only in
eGE format. This entailed such a significant increase in the number of participants of the USE.
Practically does not change the composition of the participants of the exam in the types of educational
institutions (see Table 4.7). Like last year, the overwhelming majority
tested finished general Education, and only about 2%
graduates came to the exam from educational institutions of primary or
medium vocational education.
Table4.7
Distribution of the participants of the exam in the types of educational institutions
Number
exams
Percent
A type educational institution Exams
2006 g.. 2007 g.. 2006 g.. 2007 g..
General Education institutions 86 331 66 849 95.5 95.4
Evening (replaceable) general education
institutions 487 369 0.5 0.5
Secondary school boarding school,
cadet School, boarding school with
initial flight preparation
1 144 1 369 1,3 2,0
Educational institutions of primary and
secondary vocational education 1 469 1 333 1.7 1.9
No data 958 132 1.0 0.2
Total: 90 389 70 052 100% 100%
4.3. The main results of the implementation of examination work in physics
In general, the results of the execution of examination work in 2007 were
somewhat higher than last year, but approximately at the same level as
the figures for last year. Table 4.8 shows the results of the exam in physics in 2007
on a five-point scale, and in Table 4.9 and in Fig. 4.1 - on test points in 100-
pallets. For clarity comparison, the results are presented in comparison with
previous two years.
Table4.8
Distribution of participants in the level exam
preparation(percentage of total)
Years "2" marks "P3O" 5 score "L4H" about a scale "5"
2005 10,5% 40,7% 38,1% 10,7%
2006 16,0% 41,4% 31,1% 11,5%
2007 12,3% 43,2% 32,5% 12,0%
Table4.9
Distribution of exam participants
according to the received test points in2005-2007 gG.
Year interval test scaling
mena 0-10 11-20 21-30 31-40 41-50 51-60 61-70 71-80 81-90 91-100
2005 0,09% 0,57% 6,69% 19,62% 24,27% 24,44% 16,45% 6,34% 1,03% 0,50% 68 916
2006 0,10% 0,19% 6,91% 23,65% 23,28% 19,98% 15,74% 7,21% 2,26% 0,68% 90 389
2007 0,07% 1,09% 7,80% 19,13% 27,44% 20,60% 14,82% 6,76% 1,74% 0,55% 70 052
0-10 11-20 21-30 31-40 41-50 51-60 61-70 71-80 81-90 91-100
Test score
Percentage of students who received
appropriate test score
Fig. 4.1 Distribution of participants of the exam on the received test points
Table 4.10 shows a comparison of the scales in test points in 100-point
scale with results of tasks examination In primary
Table4.10
Comparison of primary and test score intervals in2007 year
Scale interval
test points 0-10 11-20 21-30 31-40 41-50 51-60 61-70 71-80 81-90 91-100
Scale interval
primary points 0-3 4-6 7-10 11-15 16-22 23-29 30-37 38-44 45-48 49-52
For obtaining 35 points (score 3, primary score - 13) tested
it was enough to answer the 13 most simple questions first part
work. To score 65 points (estimate 4, primary score - 34), a graduate must
was, for example, to answer 25 tasks with a choice of response, solve three of four
tasks with a brief answer, and still cope with two high-level tasks
difficulties. Those who received 85 points (estimate 5, primary score - 46), practically
ideal perfectly performed the first and second part of the work and solved at least four tasks.
third parts.
The best of the best (interval from 91 to 100 points) is necessary not only
freely navigate in all matters of the school courses of physics, but also practically
do not even allow technical errors. So, for obtaining 94 points (primary score
- 49) It was possible to "not once" only 3 primary scores, allowing, for example,
arithmetic errors in solving one of the tasks of a high level of complexity
and make mistakes in response to two any questions with the choice of answer.
Unfortunately, this year did not observe the growth of the number of graduates who scored
by the results of the USE In physics, the maximum possible score. Table 4.11
the number of 100-bullfits over the past four years is given.
Table4.11
Number of tests, scored according to the results of the exam100 points
Year 2004 2005, 2006, 2007
Number of students 6 23 33 28
This year's leaders - 27 young men and only one girl (Romanova A.I. From
Novovoronezhskaya School No. 1). Like last year, among graduates Lyceum № 153
ufa - immediately two students who scored 100 points. The same results (two 100-
polennik) achieved a gymnasium number 4. A.S. Pushkin in Yoshkar-Ola.
Molecules are very small, conventional molecules cannot be viewed even into the strongest optical microscope - but some parameters of molecules can be considered quite accurate (mass), and some will only get very roughly evaluating (sizes, speed), and it would also be good to understand what is "size Molecules "and about which" speed of the molecule "we speak. So, the mass of the molecule is like "Mass of one pray" / "The number of molecules in the mole." For example, for the water molecule M \u003d 0.018 / 6 · 1023 \u003d 3 · 10-26 kg (it is possible to calculate - the number of Avogadro is known with good accuracy, and molar Mass Any molecule is easy to find).
Assessment of the size of the molecule begins with the question of what is considered its size. Now, if she were perfectly polished by a cube! However, she is not a cube, and not a ball and in general she has no clearly defined borders. How to be in such cases? Let's start published. Let us estimate the size of a more familiar object - a schoolboy. We all saw schoolchildren, we will take a mass of the middle schoolchild (and then we'll see if this choice is influenced by the result), the density of the schoolchildren is approximately like the water (remember that it is necessary to breathe air, and then you can "hang" In the water, plunging almost completely, and if you exhale, you immediately start sinking). Now you can find a schoolchildren: V \u003d 60/1000 \u003d 0.06 cubic meters. meter. If now to accept that the schoolboy has the shape of the cube, then its size is like a cubic root from the volume, i.e. Approximately 0.4 m. This is the size of the size - less growth (size "in height"), more thickness (size "in the depth"). If we don't know anything about the shape of the schoolchild's body, we will not find anything better than this answer (instead of the cube you could take the ball, but the answer would turn out to be about the same, and the diameter of the ball is more complicated than the cube's edge). But if we have additional information (from the analysis of photos, for example), then the answer can be made much more reasonable. Let it be known that the "width" of the schoolchildren on average four times less than its height, and its "depth" - even three times less. Then H * N / 4 * H / 12 \u003d V, hence H \u003d 1.5 m (there is no point in making a more accurate calculation of such a poorly defined value, focus on the possibility of a calculator in such a "calculation" is simply illiterately!). We received a completely reasonable assessment of the growth of a schoolboy if we took a lot of about 100 kg (and such schoolchildren are!), We get about 1.7 - 1.8 m - also quite reasonable.
We now estimate the size of the water molecule. We will find the volume that falls on one molecule in the "liquid water" - in it the molecules are tightly packed (it is stronger to each other than in solid, "ice" condition). Mol water has a lot of 18 g, its volume is 18 cubic meters. Santimeters. Then one molecule accounts for V \u003d 18 · 10-6 / 6 · 1023 \u003d 3 · 10-29 m3. If we do not have information about the form of a water molecule (or - if we do not want to take into account the complex form of molecules), the easiest way to consider it a cube and size to find exactly the way we have just found the size of a cubic schoolboy: d \u003d (v) 1/3 \u003d 3 · 10-10 m. That's all! It is possible to estimate the influence of the form of sufficiently complex molecules on the result of the calculation, for example, as follows: to calculate the size of gasoline molecules, counting the molecules of cubes - and then carry out an experiment, looking at the spot area from the gasoline surface on the water surface. Considering the film "Liquid surface with a thickness of one molecule" and knowing a lot of drops, you can compare the dimensions obtained by these two methods. Very instructive result will be the result!
The idea used is suitable for a completely different calculation. We estimate the average distance between adjacent sparse gas molecules for a specific case - nitrogen at a pressure of 1 atm and a temperature of 300K. To do this, we find the volume that in this gas falls on one molecule, and then everything will turn out simply. So, we take mole of nitrogen under these conditions and we will find the amount of the portion specified in the condition, and then we split this volume by the number of molecules: V \u003d R · T / P · NA \u003d 8.3 · 300/105 · 6 · 1023 \u003d 4 · 10 -26 m3. We assume that the volume is divided into tightly packaged cubic cells, and each molecule "on average" sits in the center of its cell. Then the average distance between adjacent (nearest) molecules is equal to the edge of the cubic cell: d \u003d (v) 1/3 \u003d 3 · 10-9 m. It can be seen that the gas is cut - with this ratio between the dimensions of the molecule and the distance between the "neighbors" the molecules themselves It occupy rather small - approximately 1/1000 part is the volume of the vessel. In this case, we also conducted a calculation very approximately - such not too specific values \u200b\u200bas "the average distance between adjacent molecules" there is no point in counting more accurately.
Gas laws and basics of MTKS.
If the gas is quite damaged (and this is a common thing, it most often has to be dealt with precious gases), then almost any calculation is done using a formula that binds the pressure P, volume V, the amount of gas ν and temperature T is the famous "equation The state of the ideal gas "P · V \u003d ν · r · t. How to find one of these quantities, if all others are specified, it is completely simple and understandable. But it is possible to formulate the task so that the question will be about any other value - for example, about the gas density. So, the task: to find the nitrogen density at a temperature of 300K and a pressure of 0.2 atm. I solve it. Judging by the condition of gas rather rarefied (air consisting of 80% of nitrogen and with significantly higher pressure can be considered rarellied, we breathe fluently and easily through it), and if it were and not so - there are still different formulas No - we use this, favorite. The condition is not given the volume of any portion of gas, we will set it yourself. Take 1. cubic meter Nitrogen and find the amount of gas in this amount. Knowing the molar mass of nitrogen M \u003d 0.028 kg / mol, we will find a lot of this portion - and the task is solved. The amount of gas ν \u003d p · v / r · t, the mass m \u003d ν · m \u003d m · p · v / r · t, hence the density ρ \u003d m / v \u003d m · p / r · t \u003d 0,028 · 20,000 ( 8.3 · 300) ≈ 0.2 kg / m3. We selected the volume did not enter the answer, we chose it to concrete it - it's easier to argue, because it will not necessarily imagine that the volume can be anything, and the density will be the same. However, it is possible to figure out - "Taking the volume, say, five times more, we will increase exactly five times the amount of gas, therefore, no matter how much to take, the density will be the same. It was possible to simply rewrite the favorite formula, substituting the expression to it for the amount of gas through a lot of gas portions and its molar mass: ν \u003d m / m, then the ratio M / V \u003d \u200b\u200bM · P / R · T is immediately expressed, and this is the density . It was possible to take mol of gas and find the volume occupied by it, after which the density is immediately located, because the mass of praying is known. In general, the simpler task, the more equal and beautiful ways to solve it ...
Here is another task where the question may seem unexpected: to find an air pressure difference at an altitude of 20 m and at a height of 50 m above the ground level. Temperature 00C, pressure 1 atm. Solution: If we find air density ρ under these conditions, then the pressure difference Δp \u003d ρ · g · ΔH. The density is found in the same way as in the previous task, the complexity is only that air is a mixture of gases. Considering that it consists of 80% nitrogen and 20% oxygen, we will find a mass of the mixture: m \u003d 0.8 · 0.028 + 0.2 · 0.032 ≈ 0.029 kg. The volume occupied by this mile, V \u003d R · T / P and the density is found as the ratio of these two values. Further everything is clear, the answer will be approximately 35 Pa.
Gas density will have to calculate when it is located, for example, lifting force balloon The specified volume, when calculating the amount of air in the scuba ramp cylinders, necessary for breathing under water over a known time, when calculating the amount of the osaks necessary for the transport of a given number of mercury vapors through the desert and in many other cases.
But the challenge is more comprehensive: the electric kettle pins noisily on the table, the power consumption is 1000 W, kp. Heater 75% (the rest "leaves" into the surrounding space). From the nose - the "nose" area of \u200b\u200b1 cm2 - a steam jet will fly out, evaluate the gas velocity in this jet. All the necessary data take from the tables.
Decision. We assume that saturated pairs are formed in the teapot over the water, then the rod of a saturated water vapor flies out of the nose with + 1000c. The pressure of such a couple is 1 atm, it is easy to find its density. Knowing the power to evaporate p \u003d 0.75 · p0 \u003d 750 W and the specific heat of the vaporization (evaporation) r \u003d 2300 kJ / kg, we will find a seam mass formed during τ: m \u003d 0.75r0 · τ / r. We know density, then it is easy to find the amount of this amount of steam. The rest is already clear - imagine this volume in the form of a column with a cross-sectional area of \u200b\u200b1 cm2, the length of this column divided by τ and gives us the departure rate (this length flies in a second). So, the speed of departure jets from the nose of the kettle V \u003d m / (ρ · s · τ) \u003d 0.75p0 · τ / (r · ρ · s · τ) \u003d 0.75p0 · r · t / (r · p · m · S) \u003d 750 · 8.3 · 373 / (2.3 · 106 · 1 · 105 · 0.018 · 1 · 10-4) ≈ 5 m / s.
(c) Zilberman A. R.
Molecular physics is easy!
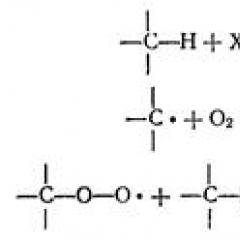
Molecules interaction forces
All substance molecules interact with each other forces of attraction and repulsion.
Proof of the interaction of molecules: the phenomenon of wetting, resistance to compression and stretching, low compressibility of solids and gases, etc.
The cause of the interaction of molecules is the electromagnetic interactions of charged particles in the substance.
How to explain it?
The atom consists of a positively charged kernel and a negatively charged electronic shell. The charge of the nucleus is equal to the total charge of all electrons, so in general the atom is electrically neutral.
The molecule consisting of one or several atoms is also electrically neutral.
Consider the interaction between molecules on the example of two fixed molecules.
Between bodies in nature, gravitational and electromagnetic forces may exist.
Since the masses of molecules are extremely small, negligible forces of gravitational interaction between molecules can not be considered.
At very large distances of electromagnetic interaction between molecules, too, no.
But, with a decrease in the distance between molecules molecules, they begin to navigate so that their parties addressed to each other will have different charges on the sign (as a whole, the molecules remain neutral), and the forces of attraction arise between molecules.
With an even greater reduction in the distance between molecules, repulsion forces arise as the result of the interaction of negatively charged electronic shells of molecules atoms.
As a result, the amount of attraction and repulsion forces acts on the molecule. At large distances, the force of attraction prevails (at a distance of 2-3 diameters of the molecule, the attraction as possible), at low distances, the repulsion force.
There is such a distance between molecules, on which the attraction force becomes equal to the repulsion forces. This position of molecules is called a stable equilibrium position.
Molecules associated with electromagnetic forces on each other and the molecule has potential energy.
In the position of a stable equilibrium, the potential energy of molecules is minimal.
In the substance, each molecule interacts simultaneously with many adjacent molecules, which also affects the magnitude of the minimum potential energy of molecules.
In addition, all substance molecules are in continuous motion, i.e. Possess kinetic energy.
Thus, the structure of the substance and its properties (solid, liquid and gaseous bodies) are determined by the relationship between the minimum potential energy of the interaction of molecules and the reserve of the kinetic energy of the thermal motion of molecules.
The structure and properties of solid, liquid and gaseous bodies
The structure of the bodies is explained by the interaction of body particles and the nature of their thermal motion.
Solid
Solid bodies have a constant shape and volume, almost incompressible.
The minimum potential energy of the interaction of molecules is greater than the kinetic energy of molecules.
Strong interaction of particles.
The thermal motion of molecules in the solid body is expressed only by oscillations of particles (atoms, molecules) near the position of a stable equilibrium.
Due to the large forces of attraction of the molecule, it is practically unable to change their position in the substance, this explains the invariability of the volume and form of solid bodies.
Most solid bodies have a particle arrangement ordered in space, which form the right crystal lattice. Particles of substance (atoms, molecules, ions) are located in the vertices - nodes of the crystal lattice. The nodes of the crystal lattice coincide with the position of the resistant equilibrium of the particles.
Such solid bodies are called crystalline.
Liquid
The fluids have a certain volume, but do not have their own form, they take the shape of the vessel in which there are.
The minimum potential energy of the interaction of molecules is comparable to the kinetic energy of molecules.
Weak particle interaction.
The thermal movement of molecules in the liquid is expressed by oscillations near the position of a stable equilibrium inside the volume provided by the molecule of its neighbors
Molecules cannot freely move throughout the volume of the substance, but it is possible to transitions of molecules to neighboring places. This explains fluid flow, the ability to change its form.

In liquids, the molecule is quite firmly connected with each other forces of attraction, which explains the invariance of the volume of the liquid.
In the fluid, the distance between molecules is approximately the diameter of the molecule. With a decrease in the distance between molecules (squeezing fluid), the repulsion force increases sharply, therefore the liquids are incompressible.
In terms of its structure and nature of the thermal motion of the liquid, the intermediate position between solid bodies and gases occupy.
Although the difference between liquid and gas is much larger than between liquid and solid body. For example, when melting or crystallization, the volume of the body varies many times less than when evaporation or condensation.
Gazes do not have a constant volume and occupy the entire volume of the vessel in which they are located.
The minimum potential energy of the interaction of molecules is less than the kinetic energy of molecules.
Particles of the substance practically do not interact.
Gases are characterized by a complete disorder of the location and movement of molecules.